
A vicinal diol has two hydroxyl groups on the same carbon atoms.
a. True
b. False
c. Ambiguous
d. None of the above
Answer
596.7k+ views
Hint: Vicinal positions are known as adjacent positions of a compound (means on adjacent atoms). And diols are alcohol groups (- OH).
Complete answer: We know that a diol is a chemical compound which contains two hydroxyl groups (−OH groups).
Some classes of diols:
Geminal diols: Geminal diols have two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. Below given is the example of geminal diols:
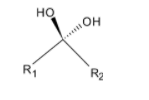
Vicinal diols: In a vicinal diol, the two hydroxyl groups occupy vicinal positions, they are attached to adjacent atoms. These compounds are called glycols. In a vicinal diol, the two hydroxyl groups occupy vicinal positions, that is, they are attached to adjacent atoms. These compounds are called glycols.
Diols such as ethylene glycol are used as co-monomer in polymerization reactions forming polymers. Below given is the structure of vicinal diol:
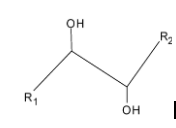
Some other diols are also there which have diols at 1,3 position, 1,4 position etc.
So, from the above explanation we can say that the given statement is ‘False’.
Complete answer: We know that a diol is a chemical compound which contains two hydroxyl groups (−OH groups).
Some classes of diols:
Geminal diols: Geminal diols have two hydroxyl groups bonded to the same atom. These species arise by hydration of the carbonyl compounds. Below given is the example of geminal diols:

Vicinal diols: In a vicinal diol, the two hydroxyl groups occupy vicinal positions, they are attached to adjacent atoms. These compounds are called glycols. In a vicinal diol, the two hydroxyl groups occupy vicinal positions, that is, they are attached to adjacent atoms. These compounds are called glycols.
Diols such as ethylene glycol are used as co-monomer in polymerization reactions forming polymers. Below given is the structure of vicinal diol:

Some other diols are also there which have diols at 1,3 position, 1,4 position etc.
So, from the above explanation we can say that the given statement is ‘False’.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




