
A white shirt has a yellow stain of curry. When soap is rubbed on this shirt during washing, the yellow stain turns reddish-brown. On rinsing the shirt with plenty of water, the reddish-brown stain turns yellow again.
(a) Name the natural indicator present in curry stain.
(b) Explain the change in colour of this indicator which takes place during washing and rinsing the shirt.
(c) What is the nature of soap (acidic/basic) as shown by the indicator present in the curry?
Answer
573.6k+ views
Hint: Turmeric is used in making curry and it is yellow in colour. When turmeric is put into an acidic solution, its colour does not change. If turmeric is put into a neutral condition, its colour still does not change. When turmeric is put into a basic solution, the yellow colour changes to red.
Complete step by step answer:
One of the most common ingredients used in curry is Turmeric. It is a yellow coloured powder derived from its flower plant. Turmeric has many uses besides an ingredient in food, it acts as an indicator with acidic and basic solutions. The molecule is turmeric which is responsible for this behaviour is known as Curcumin.
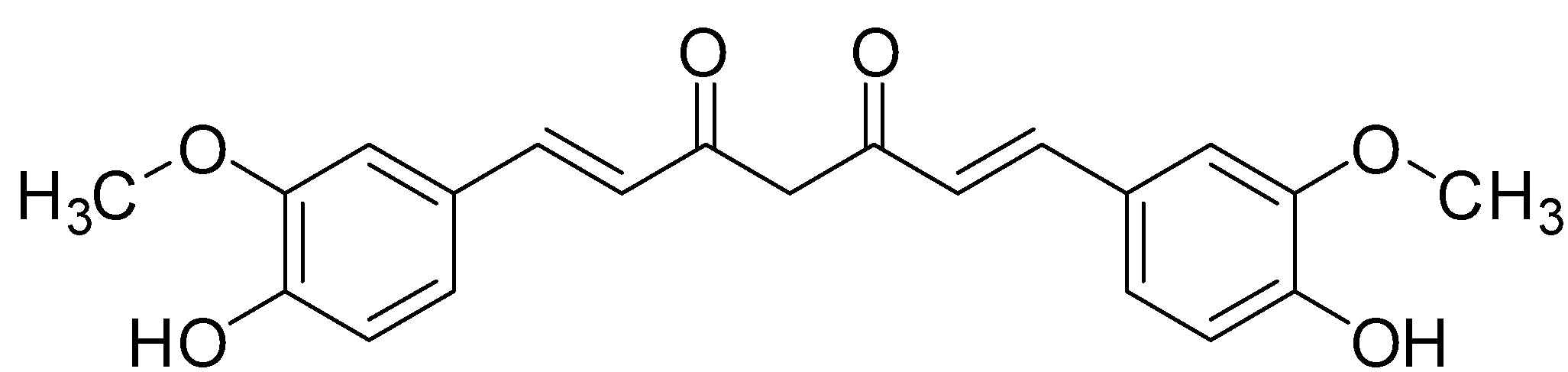
This is the structure of the molecule, Curcumin which is responsible for the yellow colour of the turmeric powder. The Nature of Turmeric as an Indicator is due to the presence of this molecule. Let us understand two words which we will be needing while explaining the Colour due to the Curcumin structure. A chromophore is a small molecule which is responsible for imparting colour to the structure. These are small molecules which have a double bond. Double bonds are formed due to a π bond, and hence these molecules can be said to have π electrons. For example: [- N {O_2}, - {N_2}, - CO] An Auxochrome is a group which is responsible for increasing the intensity of colour on a molecule. An Auxochrome in itself does not impart colour, and hence a molecule with only an auxochrome won’t be coloured. For example: $ - OH, - OC {H_3}, - N {H_2} $ in the structure for Curcumin the Chromophore, or the molecule responsible for the yellow colour is the diketone group, $ - CO$ and the auxochrome is the $ - OC {H_3}, - OH$ Groups. Curcumin retains its structure in neutral and acidic conditions but when the $pH geqslant 8$ then the molecule undergoes tautomerism Due to the shift of a Hydrogen atom
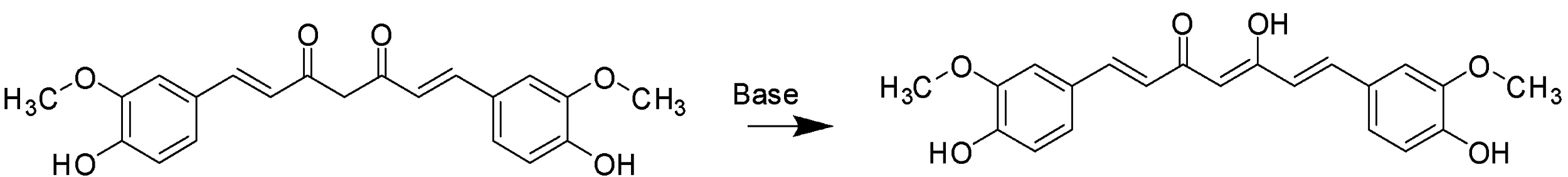
The New structure formed is a keto- enol molecule, which has a double bond adjacent to an alcohol group next to a ketone group. This structure has only one ketone group now and the number of auxochrome groups increases. The Keto-Enol group is red in colour. Hence, When Curcumin is in a basic solution, it undergoes tautomerism to form a keto-enol structure which changes its colour from yellow to red. Hence, from the information understood from above, we can say that,
1. The Natural Indicator present in the curry stain is called Turmeric
2. When it is washed with Soap it changes its colour to red, this means that the soap is basic in nature. The Curcumin molecule in Basic condition forms a red coloured keto-enol tautomer. When it is washed with water, the soap is removed and we get the original structure of Curcumin back.
3. Since, the colour of Turmeric stain changes to red, as we have understood above, now we know that Turmeric colour changes from yellow to red in basic conditions and hence, the soap is basic.
Note: Turmeric is not used as a lab indicator since its working range is very less. However, it can be used to determine if the substance is acidic or basic but cannot be used for quantitative purposes.
Turmeric also acts as an anti-oxidant and has Anti-Cancer properties. However, Since Turmeric can be easily metabolised in the body, very little amount of turmeric actually reaches the desired site in the body and the rest of it is metabolised rapidly. Hence, to be able to utilise the anticancer property of Turmeric, there needs to be a method for its controlled release in the body.
Complete step by step answer:
One of the most common ingredients used in curry is Turmeric. It is a yellow coloured powder derived from its flower plant. Turmeric has many uses besides an ingredient in food, it acts as an indicator with acidic and basic solutions. The molecule is turmeric which is responsible for this behaviour is known as Curcumin.

This is the structure of the molecule, Curcumin which is responsible for the yellow colour of the turmeric powder. The Nature of Turmeric as an Indicator is due to the presence of this molecule. Let us understand two words which we will be needing while explaining the Colour due to the Curcumin structure. A chromophore is a small molecule which is responsible for imparting colour to the structure. These are small molecules which have a double bond. Double bonds are formed due to a π bond, and hence these molecules can be said to have π electrons. For example: [- N {O_2}, - {N_2}, - CO] An Auxochrome is a group which is responsible for increasing the intensity of colour on a molecule. An Auxochrome in itself does not impart colour, and hence a molecule with only an auxochrome won’t be coloured. For example: $ - OH, - OC {H_3}, - N {H_2} $ in the structure for Curcumin the Chromophore, or the molecule responsible for the yellow colour is the diketone group, $ - CO$ and the auxochrome is the $ - OC {H_3}, - OH$ Groups. Curcumin retains its structure in neutral and acidic conditions but when the $pH geqslant 8$ then the molecule undergoes tautomerism Due to the shift of a Hydrogen atom

The New structure formed is a keto- enol molecule, which has a double bond adjacent to an alcohol group next to a ketone group. This structure has only one ketone group now and the number of auxochrome groups increases. The Keto-Enol group is red in colour. Hence, When Curcumin is in a basic solution, it undergoes tautomerism to form a keto-enol structure which changes its colour from yellow to red. Hence, from the information understood from above, we can say that,
1. The Natural Indicator present in the curry stain is called Turmeric
2. When it is washed with Soap it changes its colour to red, this means that the soap is basic in nature. The Curcumin molecule in Basic condition forms a red coloured keto-enol tautomer. When it is washed with water, the soap is removed and we get the original structure of Curcumin back.
3. Since, the colour of Turmeric stain changes to red, as we have understood above, now we know that Turmeric colour changes from yellow to red in basic conditions and hence, the soap is basic.
Note: Turmeric is not used as a lab indicator since its working range is very less. However, it can be used to determine if the substance is acidic or basic but cannot be used for quantitative purposes.
Turmeric also acts as an anti-oxidant and has Anti-Cancer properties. However, Since Turmeric can be easily metabolised in the body, very little amount of turmeric actually reaches the desired site in the body and the rest of it is metabolised rapidly. Hence, to be able to utilise the anticancer property of Turmeric, there needs to be a method for its controlled release in the body.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




