
(A)- Write a note on the wolf - kishner reduction.
(B)- Boiling points of carboxylic acids are higher than aldehydes and ketones of comparable molecular masses. Why?
(C)- Draw a dimer structure of ethanoic acid in vapor state.
Answer
560.4k+ views
Hint: (A)- As the name of the reaction suggested that the wolff – kishner reaction is a reduction reaction in which aldehyde & ketone reduced to the alkane.
(B)- Boiling point of most stable & strong compounds is always high.
(C)- Dimer structure is that structure in which two same molecules are present in one structure.
Complete step by step answer:
(A)- Short note on the wolff - kishner reduction reaction is as follow:
-In the initial mechanism of above reaction, ketone or aldehyde reacts with hydrazine to form hydrazone and the solvent used in this reaction is generally diethylene glycol.
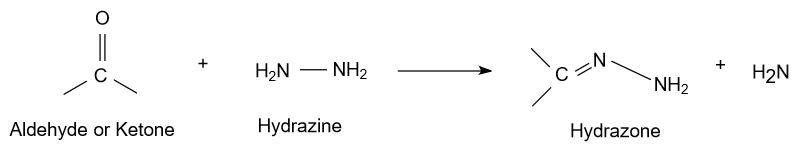
-In the latter step in the presence of basic medium final product alkanes are obtained and nitrogen gas is evolved.

(B)- Boiling point of carboxylic acid $\left( {{\text{ - COOH}}} \right)$ is higher than aldehyde $\left( {{\text{ - CHO}}} \right)$ and ketone $\left( {{\text{ - CO}}} \right)$ because the molecular weight of carboxylic acid is more than both given functional groups, so we want more energy to break that molecule.
(C)- Ethanoic acid is also known as acetic acid it is present in dimer form in the vapour phase due to the presence of hydrogen bond between two units of ethanoic acid.
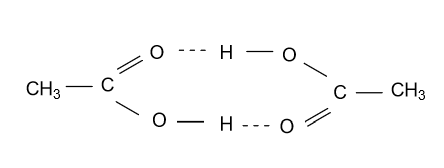
Note: In the above question you should be clear about each concept that is used in the above three points and don’t get confused in any of them. And also you should be clear in the chemical formula of functional groups.
(B)- Boiling point of most stable & strong compounds is always high.
(C)- Dimer structure is that structure in which two same molecules are present in one structure.
Complete step by step answer:
(A)- Short note on the wolff - kishner reduction reaction is as follow:
-In the initial mechanism of above reaction, ketone or aldehyde reacts with hydrazine to form hydrazone and the solvent used in this reaction is generally diethylene glycol.

-In the latter step in the presence of basic medium final product alkanes are obtained and nitrogen gas is evolved.

(B)- Boiling point of carboxylic acid $\left( {{\text{ - COOH}}} \right)$ is higher than aldehyde $\left( {{\text{ - CHO}}} \right)$ and ketone $\left( {{\text{ - CO}}} \right)$ because the molecular weight of carboxylic acid is more than both given functional groups, so we want more energy to break that molecule.
(C)- Ethanoic acid is also known as acetic acid it is present in dimer form in the vapour phase due to the presence of hydrogen bond between two units of ethanoic acid.
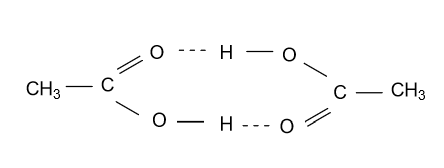
Note: In the above question you should be clear about each concept that is used in the above three points and don’t get confused in any of them. And also you should be clear in the chemical formula of functional groups.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




