
(a) Write the values of axial angles for the hexagonal crystal system.
(b) Which type of semiconductor is obtained by doping boron with silicon? Explain.
Answer
566.4k+ views
Hint:(a) The hexagonal crystal system is similar to a cylinder. However, in the hexagonal crystal system, the base is hexagonal in shape whereas in cylinder, the base is circular.
(b) Pure silicon and germanium conduct electricity to little extent. The level of electrical conductivity of pure silicon and germanium is in between that of conductors and insulators.
Complete step-by-step answer:
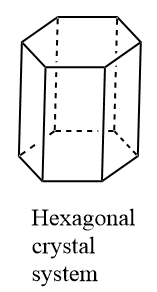
(a)The values of axial angles for a hexagonal crystal system are \[\alpha = \beta = {90^o},{\text{ }}\gamma ={120^o}\]. Thus, two angles in the hexagonal crystal system are equal to each other and the third angle is different. Two angles are right angles and the third angle is wider than right angles. Also, in a hexagonal crystal system, two sides have equal length and the third side has usually higher length.
(b)The electrical conductivity of semiconductors is in between that of conductors and insulators. Insulators do not conduct electricity at all as they have a large energy gap (band gap) between valence band and conduction band. On the other hand, conductors conduct electricity as they have little energy gap (band gap) between valence band and conduction band.
You can improve the conductivity of silicon and germanium by doping.
Doping is the process of adding a small impurity to the crystal. The purpose of this is to increase the electrical conductivity. Silicon and germanium are group 14 elements. You can dope silicon and germanium with group 15 elements such as arsenic. Due to this, the crystal will have excess electrons. You can call the doped crystal an n-type semiconductor.
You can also dope silicon and germanium with group 13 elements such as boron or indium. Due to this, the crystal will have holes. You can call the doped crystal a p-type semiconductor. Holes are electron deficiency. The symbol ‘p’ represents the flow of positive charge whereas the symbol ‘n’ represents the flow of electrons.
Pure silicon and germanium are semiconductors. P-type of semiconductor is obtained by doping boron with silicon.
Note:(a) In the hexagonal crystal system, three-unit cells share a corner. Hence, the corner angle is \[\dfrac{{{{360}^o}}}{3} = {120^o}\]
(b) You can classify semiconductors as intrinsic semiconductors and extrinsic semiconductors. You can convert an intrinsic semiconductor and extrinsic semiconductor by doping.
(b) Pure silicon and germanium conduct electricity to little extent. The level of electrical conductivity of pure silicon and germanium is in between that of conductors and insulators.
Complete step-by-step answer:
(a)The values of axial angles for a hexagonal crystal system are \[\alpha = \beta = {90^o},{\text{ }}\gamma ={120^o}\]. Thus, two angles in the hexagonal crystal system are equal to each other and the third angle is different. Two angles are right angles and the third angle is wider than right angles. Also, in a hexagonal crystal system, two sides have equal length and the third side has usually higher length.

(b)The electrical conductivity of semiconductors is in between that of conductors and insulators. Insulators do not conduct electricity at all as they have a large energy gap (band gap) between valence band and conduction band. On the other hand, conductors conduct electricity as they have little energy gap (band gap) between valence band and conduction band.
You can improve the conductivity of silicon and germanium by doping.
Doping is the process of adding a small impurity to the crystal. The purpose of this is to increase the electrical conductivity. Silicon and germanium are group 14 elements. You can dope silicon and germanium with group 15 elements such as arsenic. Due to this, the crystal will have excess electrons. You can call the doped crystal an n-type semiconductor.
You can also dope silicon and germanium with group 13 elements such as boron or indium. Due to this, the crystal will have holes. You can call the doped crystal a p-type semiconductor. Holes are electron deficiency. The symbol ‘p’ represents the flow of positive charge whereas the symbol ‘n’ represents the flow of electrons.
Pure silicon and germanium are semiconductors. P-type of semiconductor is obtained by doping boron with silicon.
Note:(a) In the hexagonal crystal system, three-unit cells share a corner. Hence, the corner angle is \[\dfrac{{{{360}^o}}}{3} = {120^o}\]
(b) You can classify semiconductors as intrinsic semiconductors and extrinsic semiconductors. You can convert an intrinsic semiconductor and extrinsic semiconductor by doping.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




