
Acetaldoxime formed from acetaldehyde is a _______ reaction.
A.nucleophilic substitution
B.nucleophilic addition
C.electrophilic substitution
D.electrophilic addition
Answer
568.5k+ views
Hint:
In this question, acetaldehyde is reacted with hydroxyl amine to produce acetaldoxime as a product. electrophiles are electron deficient and therefore accept electrons whereas nucleophiles are electron rich and have a tendency to donate electrons.
Complete step by step answer:
When an acetaldehyde reacts with hydroxyl amine it gives acetaldoxime as a product.
The reaction can be written as follows:
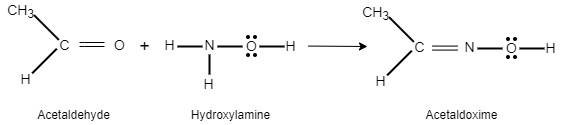
Here, the nucleophilic of the nitrogen on the hydroxylamine is increased due to the presence of oxygen. Elimination of water takes place due to proton transfer. Oximes form a mixture of geometric isomers.
Nucleophilic substitution is taking place in this reaction;
therefore, the correct option is (A) nucleophilic substitution.
Additional information:
-Nucleophilic substitution reaction is defined as the reaction in which a leaving group is replaced by an electron rich compound.
-Nucleophilic addition reaction is defined as the reaction in which double bonds and triple bonds break due to their reaction with nucleophiles.
-Electrophilic substitution reaction is defined as the reaction in which electrophile replaces a functional group.
-Electrophilic addition reaction is the reaction in which one pi bond is broken and two sigma bonds are formed.
-Acetaldehyde is defined as an organic compound that contains aldehyde as a functional group.
-Hydroxylamine is defined as an inorganic compound obtained in a crystalline form. Hydroxyl amine is used to prepare oximes. It is hygroscopic in nature.
-Acetaldoxime is a chemical compound. It is an oxime containing compound.
Note:In this reaction, water is eliminated to form oximes.
-Nucleophilic substitution reaction takes place where water is removed.
In this question, acetaldehyde is reacted with hydroxyl amine to produce acetaldoxime as a product. electrophiles are electron deficient and therefore accept electrons whereas nucleophiles are electron rich and have a tendency to donate electrons.
Complete step by step answer:
When an acetaldehyde reacts with hydroxyl amine it gives acetaldoxime as a product.
The reaction can be written as follows:

Here, the nucleophilic of the nitrogen on the hydroxylamine is increased due to the presence of oxygen. Elimination of water takes place due to proton transfer. Oximes form a mixture of geometric isomers.
Nucleophilic substitution is taking place in this reaction;
therefore, the correct option is (A) nucleophilic substitution.
Additional information:
-Nucleophilic substitution reaction is defined as the reaction in which a leaving group is replaced by an electron rich compound.
-Nucleophilic addition reaction is defined as the reaction in which double bonds and triple bonds break due to their reaction with nucleophiles.
-Electrophilic substitution reaction is defined as the reaction in which electrophile replaces a functional group.
-Electrophilic addition reaction is the reaction in which one pi bond is broken and two sigma bonds are formed.
-Acetaldehyde is defined as an organic compound that contains aldehyde as a functional group.
-Hydroxylamine is defined as an inorganic compound obtained in a crystalline form. Hydroxyl amine is used to prepare oximes. It is hygroscopic in nature.
-Acetaldoxime is a chemical compound. It is an oxime containing compound.
Note:In this reaction, water is eliminated to form oximes.
-Nucleophilic substitution reaction takes place where water is removed.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




