
Acetic acid considers as a resonance hybrid of the four structures:
Which of the above is least stable.

Answer
480.6k+ views
Hint: Acetic acid is a weak acid with the molecular formula of \[C{H_3}COOH\] . In acetic acid, the carbonyl oxygen gets a negative charge due to the more electronegativity, whereas carbonyl carbon gets a positive charge. When the carbonyl carbon is a negative charge and oxygen is a positive charge, it will be unstable.
Complete answer:
The resonance structure is the different Lewis structures of the given compound. The charges are distributed in the atoms present in the compound. The atoms, valence electrons, and bonds must be equal in all the resonance structures.
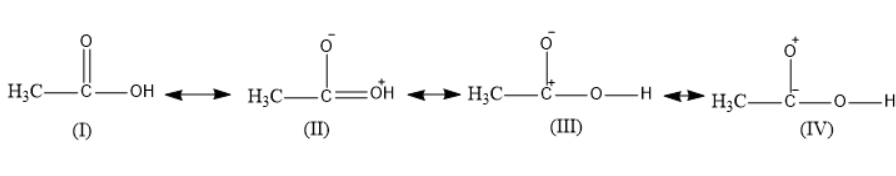
Four resonance structures of acetic acid were given.
The first structure is the normal acetic acid structure.
The second structure represents one of the resonance structures in which the carbonyl oxygen gets a negative charge and the hydroxyl oxygen gets a positive charge.
The third structure represents one of the resonance structures in which the carbonyl oxygen gets a negative charge and the carbonyl carbon gets a positive charge.
The fourth structure represents one of the resonance structures in which the carbonyl carbon gets a negative charge and the carbonyl oxygen gets a positive charge. It is the least unstable as the oxygen is more electronegative; it does not get a positive charge.
Thus, \[IV\] structure is the least stable resonance structure of acetic acid.
Note:
Electronegativity is nothing but the ability to attract the electrons towards themselves. In the periodic table, fluorine is the most electronegative element, followed by oxygen. Oxygen is more electronegative than carbon due to this reason, in the carbonyl group the oxygen atom gets a negative charge only.
Complete answer:
The resonance structure is the different Lewis structures of the given compound. The charges are distributed in the atoms present in the compound. The atoms, valence electrons, and bonds must be equal in all the resonance structures.
Four resonance structures of acetic acid were given.
The first structure is the normal acetic acid structure.
The second structure represents one of the resonance structures in which the carbonyl oxygen gets a negative charge and the hydroxyl oxygen gets a positive charge.
The third structure represents one of the resonance structures in which the carbonyl oxygen gets a negative charge and the carbonyl carbon gets a positive charge.
The fourth structure represents one of the resonance structures in which the carbonyl carbon gets a negative charge and the carbonyl oxygen gets a positive charge. It is the least unstable as the oxygen is more electronegative; it does not get a positive charge.
Thus, \[IV\] structure is the least stable resonance structure of acetic acid.
Note:
Electronegativity is nothing but the ability to attract the electrons towards themselves. In the periodic table, fluorine is the most electronegative element, followed by oxygen. Oxygen is more electronegative than carbon due to this reason, in the carbonyl group the oxygen atom gets a negative charge only.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




