
Acetic acid reacts with $PCl_{5}$ or $SOCl_{2}$ to give:
A.Acetyl chloride
B.Methyl chloride
C.Dichloroacetic acid
D.Ethanol
Answer
539.7k+ views
Hint:Acetic acid is a carboxylic acid, highly polar and exhibits hydrogen bonding as well. It is completely miscible with water. The general reaction is known as acyl substitution which is given by carboxylic acid is nucleophilic addition elimination.
Complete step-by-step answer:The compound is further converted into functional derivatives where the -OH of an acid is replaced by -Cl, -OR etc. in order to yield acid chlorides and esters. These compounds are called functional derivatives of acid as they contain acyl groups. An acid chloride is produced by the substitution of -Cl for the -OH in the presence of an acid.
Three reagents commonly used for this purpose are thionyl chloride, phosphorus trichloride and phosphorus pentachloride which has been asked in the question. Therefore, the acid chloride formed by nucleophilic addition elimination reaction on acetic acid with phosphorus pentachloride is given below.
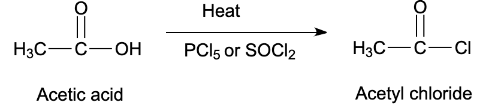
Acyl chlorides are the foremost reactive of the acid derivatives because they need good leaving group chloride ions attached to the carbonyl carbon atom by protonation. The initial step involves nucleophilic addition at the carbonyl carbon atom after this elimination leads to regeneration of the carbon-oxygen double bond and to a substitution product.
Therefore, the correct option is (A).
Note:Thionyl chloride is particularly convenient, since products formed besides the acid chloride are gases and thus can be easily separated from the acid chloride. Moreover, excess of the thionyl chloride is easily removed by distillation.
Complete step-by-step answer:The compound is further converted into functional derivatives where the -OH of an acid is replaced by -Cl, -OR etc. in order to yield acid chlorides and esters. These compounds are called functional derivatives of acid as they contain acyl groups. An acid chloride is produced by the substitution of -Cl for the -OH in the presence of an acid.
Three reagents commonly used for this purpose are thionyl chloride, phosphorus trichloride and phosphorus pentachloride which has been asked in the question. Therefore, the acid chloride formed by nucleophilic addition elimination reaction on acetic acid with phosphorus pentachloride is given below.

Acyl chlorides are the foremost reactive of the acid derivatives because they need good leaving group chloride ions attached to the carbonyl carbon atom by protonation. The initial step involves nucleophilic addition at the carbonyl carbon atom after this elimination leads to regeneration of the carbon-oxygen double bond and to a substitution product.
Therefore, the correct option is (A).
Note:Thionyl chloride is particularly convenient, since products formed besides the acid chloride are gases and thus can be easily separated from the acid chloride. Moreover, excess of the thionyl chloride is easily removed by distillation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




