
When acetone and chloroform are mixed, hydrogen bonding takes place between them. Such a liquid pair cause:
(A)Positive deviation from Raoult’s law
(B)Negative deviation from Raoult’s law
(C)No deviation from raoult’s law
(D)Cannot be predicted
Answer
579.3k+ views
Hint: To solve this question, we have to know Raoult's law. The binary solution that obeys Raoult’s law is known as the ideal solution and the binary solution that shows deviation from Raoult’s law is known as non-ideal solution.
Complete step by step solution:
In order to understand the concept behind this question we should know Raoult's law. Raoult’s law states that “The partial pressure of any volatile component of solution at any temperature, is equal to the vapour pressure of pure component multiplied by the mole fraction of the component”.
The binary solution that obeys Raoult’s law under all concentration and all temperatures is said to be the ideal solution, whereas the binary solution that shows either a positive or negative deviation from Raoult’s law is said to be a non ideal solution.
When a binary solution is a non-ideal solution, it will either show positive deviation or negative deviation. Chloroform is able to form hydrogen bonds with acetone.
Let chloroform be (A) and acetone be (B):
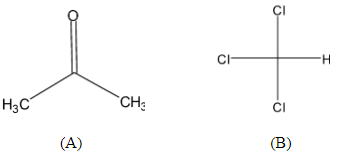
The A-A interaction and B-B interaction are weaker than A-B interaction so the tendency of the component A and B will have lower vapour pressure. The interaction between them is intermolecular hydrogen. The total vapour pressure of the mixture will be below the vapour pressure of ideal compounds due the strong hydrogen bond between compound A and B.
There will be lowering of vapor pressure from ideal solutions hence, shows negative deviation.
Thus option B is the correct answer.
Note: The vapour pressure of components will be very less than that was predicted by Raoult’s law. So the total vapour pressure curve dips in minimum. The total vapor pressure of the mixture of chloroform and acetone will be below that of vapour pressure of the pure components because the interaction between A-A and B-B is less than A-B ( Refer the molecule A and B in above mentioned diagram).
Complete step by step solution:
In order to understand the concept behind this question we should know Raoult's law. Raoult’s law states that “The partial pressure of any volatile component of solution at any temperature, is equal to the vapour pressure of pure component multiplied by the mole fraction of the component”.
The binary solution that obeys Raoult’s law under all concentration and all temperatures is said to be the ideal solution, whereas the binary solution that shows either a positive or negative deviation from Raoult’s law is said to be a non ideal solution.
When a binary solution is a non-ideal solution, it will either show positive deviation or negative deviation. Chloroform is able to form hydrogen bonds with acetone.
Let chloroform be (A) and acetone be (B):

The A-A interaction and B-B interaction are weaker than A-B interaction so the tendency of the component A and B will have lower vapour pressure. The interaction between them is intermolecular hydrogen. The total vapour pressure of the mixture will be below the vapour pressure of ideal compounds due the strong hydrogen bond between compound A and B.
There will be lowering of vapor pressure from ideal solutions hence, shows negative deviation.
Thus option B is the correct answer.
Note: The vapour pressure of components will be very less than that was predicted by Raoult’s law. So the total vapour pressure curve dips in minimum. The total vapor pressure of the mixture of chloroform and acetone will be below that of vapour pressure of the pure components because the interaction between A-A and B-B is less than A-B ( Refer the molecule A and B in above mentioned diagram).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




