
Acetone and methanol can be separated by:
A) Steam distillation
B) Fractional distillation
C) Vacuum distillation
D) None of the above
Answer
523.7k+ views
Hint: The mixture of liquids can be separated by the Distillation process. The process involves the heating of the mixture so that the component with low boiling point escapes first followed by the other. The acetone and methanol are volatile liquids. The boiling point of liquids is close to each other. Thus, a suitable and sophisticated distillation technique is used to separate the liquids.
Complete step by step answer:
The fractional distillation is employed to separate the mixture of two volatile liquids such that they have close boiling points. The fractional distillation process is used for the mixture of liquids where the difference in the boiling point of liquids is then that of the$\text{ 15 K }$. The fractional distillation is a process of continuous distillation and condensation of the components of the mixture. As the name suggests, the components are separated as the fraction. The mixture is heated such that the components start to vaporize at its boiling point and the fraction is separated. The acetone is a volatile liquid. It has a boiling point of $\text{ 5}{{\text{6}}^{\text{0}}}\text{C }$or $\text{ 330 K }$ while the methyl alcohol or the methanol boils at the $\text{ 6}{{\text{5}}^{\text{0}}}\text{C }$ or$\text{ 338K}$. The difference in the boiling point of methanol is acetone is $\text{ 8K }$. Thus, instead of using simple distillation, we opt for fractional distillation.
It is similar to the simple distillation except for the fractionating column. The fractionating column is introduced between the distillation flask (Round bottom flask) and the condenser.
When the mixture of miscible liquid is heated, the component which has the lower boiling point starts to boil first and separated first followed by the other components which have a higher boiling point.
The general apparatus is as shown below,
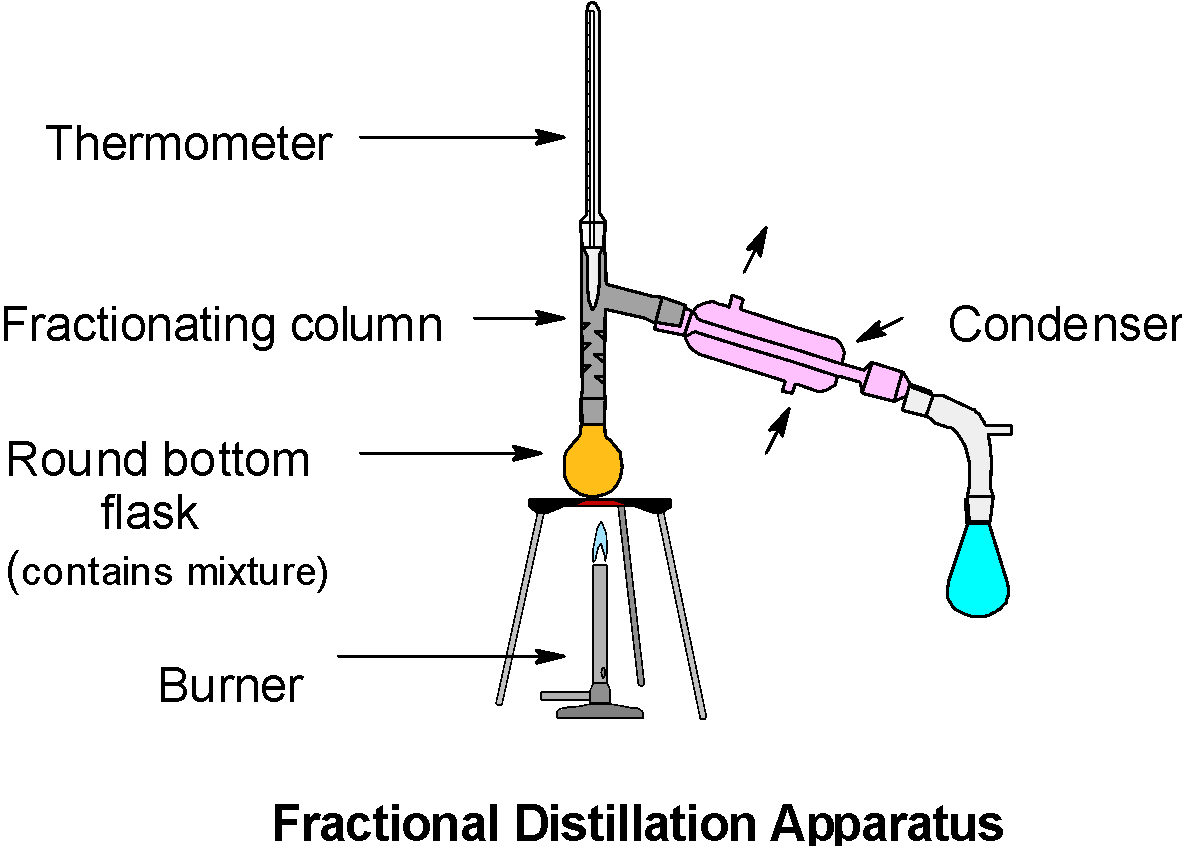
Here, the acetone has a low boiling point, it vaporizes first and undergoes the continuous condensation – vaporization cycles in the fractionating column. The mixture has a low boiling point, thus both components are likely to vaporize. However, as we increase the height of the column, the temperature at the bottom of the column decreases. So, the component which has a low boiling point (here acetone) easily escapes from the mixture and condense. Likewise, with a further increase in temperature, the methanol can be separated from the mixture as one of the fractions. Thus, the acetone and methanol can be separated by the fractional distillation.
Hence, (B) is the correct option.
Note: Note that, the acetone and methanol from a minimum boiling azeotrope (azeotrope is a mixture of liquids that have the same concentration in the liquids and vapour phase). Thus, acetone cannot be completely removed from the mixture by distillation. Thus, nowadays the extractive distillation is used in presence of selective solvents such as water.
Complete step by step answer:
The fractional distillation is employed to separate the mixture of two volatile liquids such that they have close boiling points. The fractional distillation process is used for the mixture of liquids where the difference in the boiling point of liquids is then that of the$\text{ 15 K }$. The fractional distillation is a process of continuous distillation and condensation of the components of the mixture. As the name suggests, the components are separated as the fraction. The mixture is heated such that the components start to vaporize at its boiling point and the fraction is separated. The acetone is a volatile liquid. It has a boiling point of $\text{ 5}{{\text{6}}^{\text{0}}}\text{C }$or $\text{ 330 K }$ while the methyl alcohol or the methanol boils at the $\text{ 6}{{\text{5}}^{\text{0}}}\text{C }$ or$\text{ 338K}$. The difference in the boiling point of methanol is acetone is $\text{ 8K }$. Thus, instead of using simple distillation, we opt for fractional distillation.
It is similar to the simple distillation except for the fractionating column. The fractionating column is introduced between the distillation flask (Round bottom flask) and the condenser.
When the mixture of miscible liquid is heated, the component which has the lower boiling point starts to boil first and separated first followed by the other components which have a higher boiling point.
The general apparatus is as shown below,

Here, the acetone has a low boiling point, it vaporizes first and undergoes the continuous condensation – vaporization cycles in the fractionating column. The mixture has a low boiling point, thus both components are likely to vaporize. However, as we increase the height of the column, the temperature at the bottom of the column decreases. So, the component which has a low boiling point (here acetone) easily escapes from the mixture and condense. Likewise, with a further increase in temperature, the methanol can be separated from the mixture as one of the fractions. Thus, the acetone and methanol can be separated by the fractional distillation.
Hence, (B) is the correct option.
Note: Note that, the acetone and methanol from a minimum boiling azeotrope (azeotrope is a mixture of liquids that have the same concentration in the liquids and vapour phase). Thus, acetone cannot be completely removed from the mixture by distillation. Thus, nowadays the extractive distillation is used in presence of selective solvents such as water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




