
How many $s{{p}^{2}}$ and sp- hybridized carbon atoms are present respectively in the following compound?
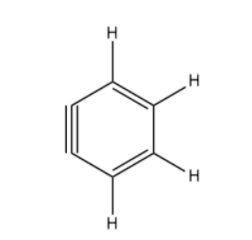
(A) 4,2
(B) 6,0
(C) 3,3
(D) 5,1

Answer
526.9k+ views
Hint: The given structure is benzyne, which is a derivative of benzene. The structural difference in benzene and benzyne is the triple bond present in the benzyne.
Complete step by step solution:
Benzyne is an example of an aryne ( -yne refers to the triple bond ). The triple bond found in benzyne structure is not an ordinary triple bond as the second $\pi $ bond results in a weak interaction of $s{{p}^{2}}$ hybrid orbitals lying in the plane of the ring. The triple bond is non-linear due to the constraints of the 6-membered benzyne ring.
Let us consider that the two triply bonded carbon atoms in a benzyne molecule are sp -hybridized, like in a typical of triply bonded carbon atoms, then there would be severe angle strain in the molecule, which causes instability of the molecule. But this molecule is not as unstable.
So, it is likely that each triply bonded carbon atom in a benzyne molecule is sp2-hybridized, in this case the two $s{{p}^{2}}$-hybridized orbitals that are not parallel to each other overlap laterally to form the pi bond that is not part of the cloud of pi electrons which lies below and above the benzene ring plane.
Hence, all the carbon atoms are $s{{p}^{2}}$ hybridized and not a single carbon atom is sp hybridized here in benzyne, so the correct option is (B) 6,0.
Note: Structure might show that the presence of triple bond i.e. 1 sigma and 2 pi bonds which leads to an assumption that the carbon atoms forming the bond are sp hybrid. But the hybridization state of these carbon atoms is $s{{p}^{2}}$ because of the above-given reason.
Complete step by step solution:
Benzyne is an example of an aryne ( -yne refers to the triple bond ). The triple bond found in benzyne structure is not an ordinary triple bond as the second $\pi $ bond results in a weak interaction of $s{{p}^{2}}$ hybrid orbitals lying in the plane of the ring. The triple bond is non-linear due to the constraints of the 6-membered benzyne ring.
Let us consider that the two triply bonded carbon atoms in a benzyne molecule are sp -hybridized, like in a typical of triply bonded carbon atoms, then there would be severe angle strain in the molecule, which causes instability of the molecule. But this molecule is not as unstable.
So, it is likely that each triply bonded carbon atom in a benzyne molecule is sp2-hybridized, in this case the two $s{{p}^{2}}$-hybridized orbitals that are not parallel to each other overlap laterally to form the pi bond that is not part of the cloud of pi electrons which lies below and above the benzene ring plane.
Hence, all the carbon atoms are $s{{p}^{2}}$ hybridized and not a single carbon atom is sp hybridized here in benzyne, so the correct option is (B) 6,0.
Note: Structure might show that the presence of triple bond i.e. 1 sigma and 2 pi bonds which leads to an assumption that the carbon atoms forming the bond are sp hybrid. But the hybridization state of these carbon atoms is $s{{p}^{2}}$ because of the above-given reason.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




