
Acetone in addition to methyl magnesium bromide forms a complex, which on decomposition with acid gives X and Mg(OH)Br. Which one of the following is X?
(A) $C{{H}_{3}}OH$
(B) ${{(C{{H}_{3}})}_{3}}COH$
(C) ${{(C{{H}_{3}})}_{2}}CHOH$
(D) $C{{H}_{3}}C{{H}_{2}}OH$
Answer
526.1k+ views
Hint: The reaction of acetone in addition to methyl magnesium bromide forms a complex followed by hydrolysis to give tertiary alcohol, in this case, it will be tertiary butyl alcohol. Here, methyl magnesium bromide is a Grignard reagent. Acetone is a ketone.
Complete step by step solution:
From your chemistry lessons, you have learned about the Grignard reagent. The Grignard reagent is a chemical compound which has the formula of R-Mg-X where 'R' is the organic group may be alkyl or aryl,'Mg' is the magnesium and X is the halogen.
Grignard reagent makes a less or more direct bond between the metal centre and a carbon centre, $RC{{H}_{2}}{{^{\delta -}}^{\delta +}}MgX$ or maybe a full-blown carbanion.
The Grignard reagent reacts with aldehyde and ketones to give alcohol as it reacts with an aldehyde to form secondary alcohol (2⁰), it gives tertiary alcohol (3⁰) when reacts with ketone and gives carboxylic acid when reacts with carbon dioxide and ethylene oxide.
In the question methyl magnesium bromide is reacting with acetone here methyl magnesium bromide is a Grignard reagent (RMgX) and it transforms the alkyl group into a strong nucleophile. In the question the alkyl group is methyl.
The pi electrons of carbonyl bonds are pushed into the oxygen to form a tetrahedral intermediate when the nucleophile attacks the electrophilic carbonyl carbon. The oxygen will get protonated and we are left with tertiary alcohol because there is no good leaving group in respect with carbonyl carbon.
So, when acetone [${{(C{{H}_{3}})}_{2}}C=O$] reacts with methyl magnesium bromide ($C{{H}_{3}}MgBr$) it forms a complex [${{(C{{H}_{3}})}_{3}}COMgBr$] which is followed by hydrolysis to give tertiary butyl alcohol [${{(C{{H}_{3}})}_{3}}COH$]
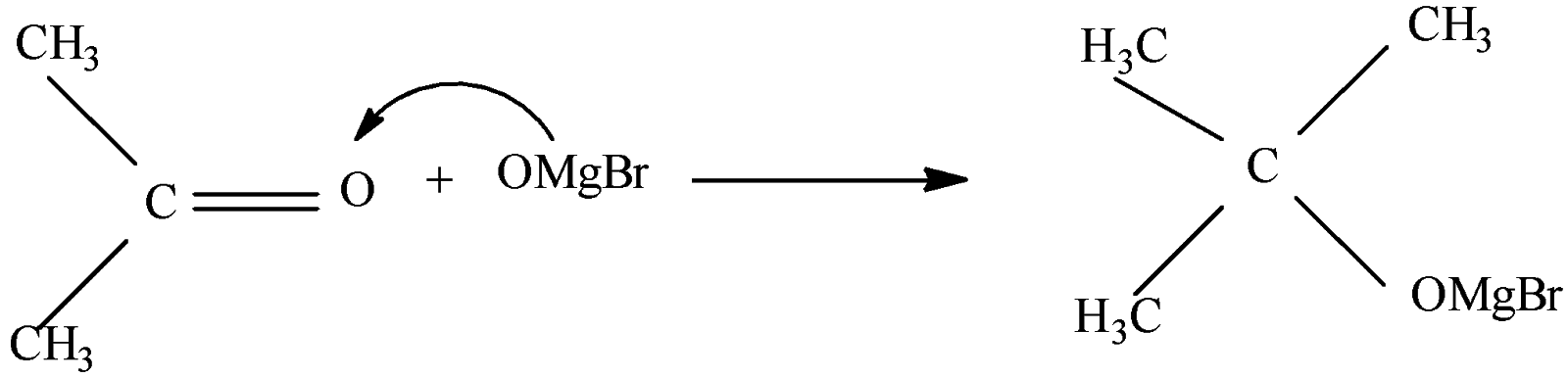
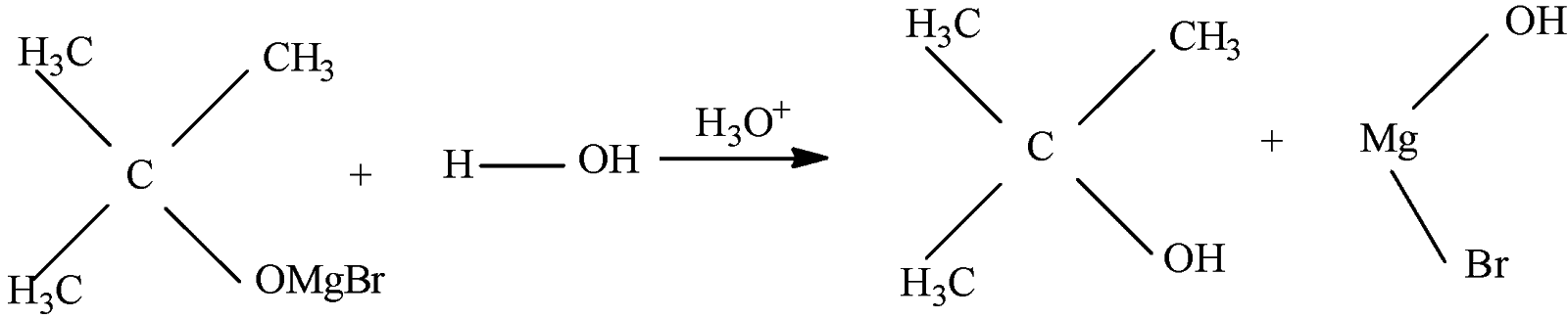
Thus the correct option will be (B).
Note: Propanone is the IUPAC name of acetone and they belong to the ketone family and that is why they form tertiary alcohol and not secondary alcohol. Acetone can be prepared by reacting methyl magnesium bromide with methyl nitrile. The Grignard reagent is important to make Carbon-Carbon bonds. Hydrolysis is a chemical breakdown of compounds when treated with water.
Complete step by step solution:
From your chemistry lessons, you have learned about the Grignard reagent. The Grignard reagent is a chemical compound which has the formula of R-Mg-X where 'R' is the organic group may be alkyl or aryl,'Mg' is the magnesium and X is the halogen.
Grignard reagent makes a less or more direct bond between the metal centre and a carbon centre, $RC{{H}_{2}}{{^{\delta -}}^{\delta +}}MgX$ or maybe a full-blown carbanion.
The Grignard reagent reacts with aldehyde and ketones to give alcohol as it reacts with an aldehyde to form secondary alcohol (2⁰), it gives tertiary alcohol (3⁰) when reacts with ketone and gives carboxylic acid when reacts with carbon dioxide and ethylene oxide.
In the question methyl magnesium bromide is reacting with acetone here methyl magnesium bromide is a Grignard reagent (RMgX) and it transforms the alkyl group into a strong nucleophile. In the question the alkyl group is methyl.
The pi electrons of carbonyl bonds are pushed into the oxygen to form a tetrahedral intermediate when the nucleophile attacks the electrophilic carbonyl carbon. The oxygen will get protonated and we are left with tertiary alcohol because there is no good leaving group in respect with carbonyl carbon.
So, when acetone [${{(C{{H}_{3}})}_{2}}C=O$] reacts with methyl magnesium bromide ($C{{H}_{3}}MgBr$) it forms a complex [${{(C{{H}_{3}})}_{3}}COMgBr$] which is followed by hydrolysis to give tertiary butyl alcohol [${{(C{{H}_{3}})}_{3}}COH$]


Thus the correct option will be (B).
Note: Propanone is the IUPAC name of acetone and they belong to the ketone family and that is why they form tertiary alcohol and not secondary alcohol. Acetone can be prepared by reacting methyl magnesium bromide with methyl nitrile. The Grignard reagent is important to make Carbon-Carbon bonds. Hydrolysis is a chemical breakdown of compounds when treated with water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




