
Acidified potassium permanganate is decolourised by
A.Bleaching powder
B.White vitriol
C.Mohr’s salt
D.Microcosmic salt
E.Laughing gas
Answer
559.5k+ views
Hint:We need to know that the potassium permanganate chemical formula is $KMn{O_4}$ , it is an inorganic compound. In the chemical industry and laboratories potassium permanganate is widely used. In the laboratories $KMn{O_4}$ is used as a strong oxidizing agent in many reactions, because potassium permanganate contains $MnO_4^ - $ ions.
Complete step by step answer:
As we know that, potassium permanganate is composed of ${K^ + }$ and $MnO_4^ - $ . Potassium permanganate is used in many titrations as an oxidizing agent.
We can draw the potassium permanganate structure as,
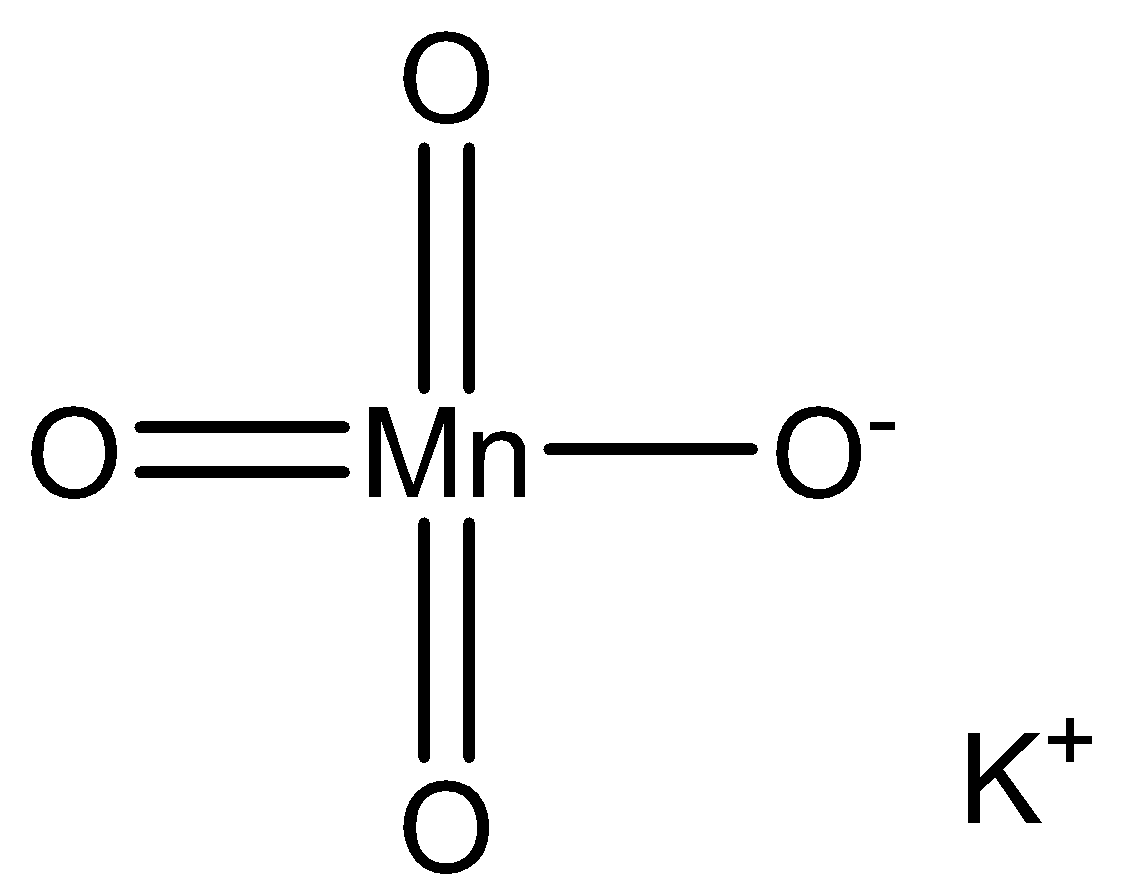
We use acidified potassium permanganate. The aicd is sulphuric acid. The acid prevents hydrolysis. Acid provides protons and it is necessary to carry a reaction. The equation for the reaction is,
\[2KMn{O_4} + 3{H_2}S{O_4} \to {K_2}S{O_4} + 2MnS{O_4} + 3{H_2}O + 5\left[ O \right]\]
Acidified potassium permanganate decolourised equation is,
$\left( {2FeS{O_4}{{(N{H_4})}_2}S{O_4}.6{H_2}O + {H_2}S{O_4} + 5\left[ O \right] \to F{e_2}{{(S{O_4})}_3} + 2{{(N{H_4})}_2}S{O_4} + 13{H_2}O} \right) \times 5$
The overall equation for above two equation is,
\[2KMn{O_4} + 10FeS{O_4}{(N{H_4})_2}S{O_4}.6{H_2}O \to K_2SO_4 + 2MnS{O_4} + 5F{e_2}{(S{O_4})_3} + 10{(N{H_4})_2}S{O_4} + 68{H_2}O\]\[{(N{H_4})_2}Fe{(S{O_4})_2}{({H_2}O)_6}\] is known as ammonium iron $(II)$ sulphate and it is used for decolourise acidified potassium permanganate.
We must need to know that the ammonium iron $(II)$ sulphate is also known as Mohr’s salt. Mohr was a German scientist.
From the above data Mohr’s salt is used to decolourise acidified potassium permanganate.
So C. Mohr’s salt is the correct answer.
Additional information:
We must need to remember that for detecting halides, oxalates, sulphates, etc. detected in qualitative analysis with the use of potassium permanganate. Potassium permanganate is also used in medicines and also used as disinfectant. Baeyer reagent is the alkaline solution of potassium permanganate.
Note:
We need to know that in the compounds of $Mn(VII)$ , potassium permanganate $(KMn{O_4})$ is the most important compound. Potassium permanganate color is purple due to the permanganate ion, $MnO_4^ - $. Potassium permanganate is reduced to manganese $(II)$ ion in acidic solution. The equation for the reaction is,
\[2KMn{O_4} + 3{H_2}S{O_4} \to {K_2}S{O_4} + 2MnS{O_4} + 3{H_2}O + 5\left[ O \right]\]
For an organic compound acidified potassium permanganate is also used as an oxidizing agent.
Complete step by step answer:
As we know that, potassium permanganate is composed of ${K^ + }$ and $MnO_4^ - $ . Potassium permanganate is used in many titrations as an oxidizing agent.
We can draw the potassium permanganate structure as,

We use acidified potassium permanganate. The aicd is sulphuric acid. The acid prevents hydrolysis. Acid provides protons and it is necessary to carry a reaction. The equation for the reaction is,
\[2KMn{O_4} + 3{H_2}S{O_4} \to {K_2}S{O_4} + 2MnS{O_4} + 3{H_2}O + 5\left[ O \right]\]
Acidified potassium permanganate decolourised equation is,
$\left( {2FeS{O_4}{{(N{H_4})}_2}S{O_4}.6{H_2}O + {H_2}S{O_4} + 5\left[ O \right] \to F{e_2}{{(S{O_4})}_3} + 2{{(N{H_4})}_2}S{O_4} + 13{H_2}O} \right) \times 5$
The overall equation for above two equation is,
\[2KMn{O_4} + 10FeS{O_4}{(N{H_4})_2}S{O_4}.6{H_2}O \to K_2SO_4 + 2MnS{O_4} + 5F{e_2}{(S{O_4})_3} + 10{(N{H_4})_2}S{O_4} + 68{H_2}O\]\[{(N{H_4})_2}Fe{(S{O_4})_2}{({H_2}O)_6}\] is known as ammonium iron $(II)$ sulphate and it is used for decolourise acidified potassium permanganate.
We must need to know that the ammonium iron $(II)$ sulphate is also known as Mohr’s salt. Mohr was a German scientist.
From the above data Mohr’s salt is used to decolourise acidified potassium permanganate.
So C. Mohr’s salt is the correct answer.
Additional information:
We must need to remember that for detecting halides, oxalates, sulphates, etc. detected in qualitative analysis with the use of potassium permanganate. Potassium permanganate is also used in medicines and also used as disinfectant. Baeyer reagent is the alkaline solution of potassium permanganate.
Note:
We need to know that in the compounds of $Mn(VII)$ , potassium permanganate $(KMn{O_4})$ is the most important compound. Potassium permanganate color is purple due to the permanganate ion, $MnO_4^ - $. Potassium permanganate is reduced to manganese $(II)$ ion in acidic solution. The equation for the reaction is,
\[2KMn{O_4} + 3{H_2}S{O_4} \to {K_2}S{O_4} + 2MnS{O_4} + 3{H_2}O + 5\left[ O \right]\]
For an organic compound acidified potassium permanganate is also used as an oxidizing agent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




