
In electron dot structure, the valence shell electrons are represented by crosses or dots.
(a) The atomic number of chlorine is 17. Write its electronic configuration.
(b) Draw the electron dot structure of a chlorine molecule.
Answer
531k+ views
Hint: In the given question firstly we have to define what exactly is the chlorine and then we can give the proper definition and the characteristics of it. Although we can say that we know that the main points among the following is that it should have a total of 17 electrons in its neutral state.
Complete step by step answer:
Copper and correct explanation of the chlorine and the different characteristics of the element both physical and chemical.
Chlorine is the chemical element with the chemical symbol as Cl and the atomic number as 17. It is also the second-lightest of the halogens and also appears between fluorine and bromine in the periodic table. Therefore we can say that its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the Pauling scale, behind only oxygen and fluorine.
(a) Electronic configuration of chlorine: 2.8,7
(b) Electron dot structure of chlorine molecule is given below:
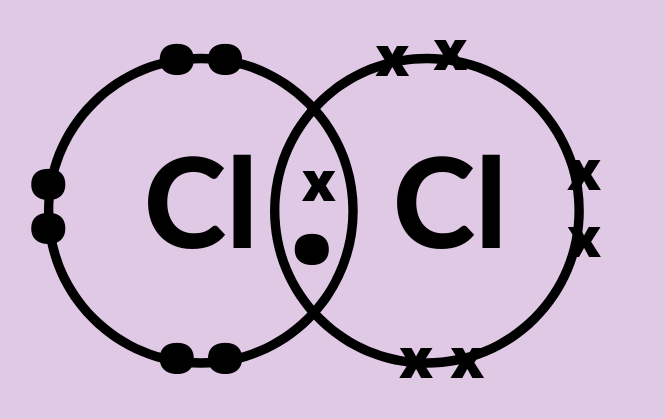
Note: The most common compound of chlorine, sodium chloride, has been known since ancient times; archaeologists have found evidence that rock salt was used as early as 3000 BC and brine as early as 6000 BC. Its importance in food was very well known in classical antiquity.
Complete step by step answer:
Copper and correct explanation of the chlorine and the different characteristics of the element both physical and chemical.
Chlorine is the chemical element with the chemical symbol as Cl and the atomic number as 17. It is also the second-lightest of the halogens and also appears between fluorine and bromine in the periodic table. Therefore we can say that its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the Pauling scale, behind only oxygen and fluorine.
(a) Electronic configuration of chlorine: 2.8,7
(b) Electron dot structure of chlorine molecule is given below:

Note: The most common compound of chlorine, sodium chloride, has been known since ancient times; archaeologists have found evidence that rock salt was used as early as 3000 BC and brine as early as 6000 BC. Its importance in food was very well known in classical antiquity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




