
What is the action of alcoholic $\text{ KOH }$ on ethyl bromide and 2-chlorobutane?
Answer
529.7k+ views
Hint: When haloalkanes with $\text{ }\beta -\text{ }$ hydrogen atoms are boiled with an alcoholic solution of potassium hydroxide, they undergo the elimination of hydrogen halide $\text{ }\left( \text{HX} \right)\text{ }$ resulting in the formation of alkenes. These reactions are known as the dehydrohalogenation or $\text{ }\beta -\text{ }$ elimination reactions. The major product is obtained by Saytzeff’s rule.
Complete step by step answer:
When haloalkanes with $\text{ }\beta -\text{ }$ hydrogen atoms are boiled with an alcoholic solution of potassium hydroxide, they undergo the elimination of hydrogen halide $\text{ }\left( \text{HX} \right)\text{ }$ resulting in the formation of alkenes. The general reaction of the dehydrohalogenation of the haloalkanes is as shown below,
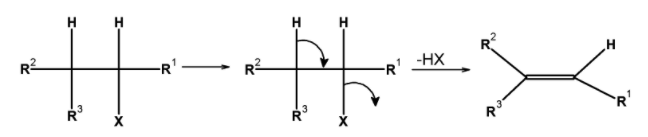
These reactions are called the $\text{ }\beta -\text{ }$ elimination reactions because the hydrogen atom present at the $\text{ }\beta -\text{ }$ position of haloalkanes (i.e. at the carbon atom next to that which carries the halogen) is removed.
a) Ethyl bromide reaction with alc.$\text{ KOH }$:
The ethyl bromide reacted with the alcoholic potassium hydroxide $\text{ KOH }$. Here, bromine (halogen group) is at the $\text{ }\alpha \text{ }$ position and hydrogen is at the $\text{ }\beta \text{ }$ position. When boiled with
$\text{ KOH }$, hydrogen atom transfers its electron pair to the adjacent carbon-carbon bond, and bromide is removed from the molecule. This forms a double bond between the alpha and beta carbon atom and gives ethane as the product.
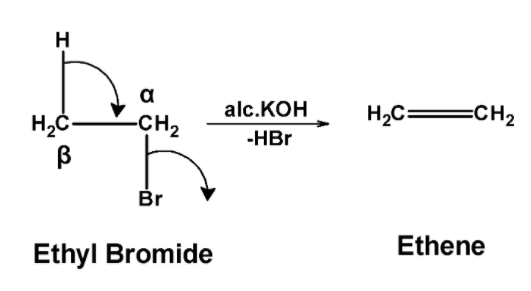
Thus, ethyl bromide on reaction with alcohol $\text{ KOH }$ gives ethene.
b) 2-chlorobutane reaction with alc.$\text{ KOH }$:
The 2-chlorobutane reacts with the alcoholic potassium hydroxide $\text{ KOH }$. Here, chlorine (halogen group) is at the $\text{ }\alpha \text{ }$ position and hydrogen is at the $\text{ }\beta \text{ }$ position. When boiled with
$\text{ KOH }$, hydrogen atom transfers its electron pair to the adjacent carbon-carbon bond, and bromide is removed from the molecule. This forms a double bond between the alpha and beta carbon atom and gives ethane as the product.
The 2-chlorobutane has the chloro group at the second carbon atom thus there are two $\text{ }\beta \text{ }$ positions. In the case of haloalkanes which has more than one $\text{ }\beta -\text{ }$ position can eliminate the hydrogen halide in two different ways, then that alkene will be preferred in which carbon atoms joined by the double bond are maximally alkylated. This rule is called the Saytzeff’s rule.
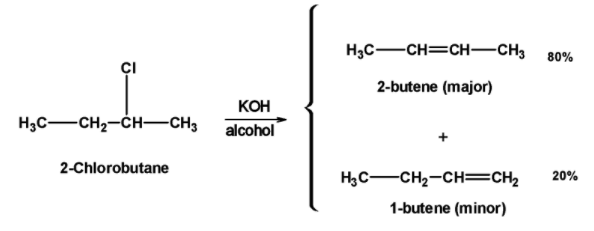
Thus, 2-chloro butane on reaction with alcohol $\text{ KOH }$ gives 2-butene as the major product. This is because the double-bonded carbons are linked to two methyl groups thus it is highly alkylated compared to 1-butene.
Note: Note that, if the substituted alkene formed during the elimination is capable of showing the cis-trans isomerism, then trans –alkene is always preferably formed as the major product because of its greater stability. For example here major product 2-butene exists in two isomers cis and trans and trans-isomer will be greater in amount. Thus we can say that $\text{ }\beta -\text{ }$ elimination of secondary halide results in three product major product (cis + trans isomers) and a minor product.
Complete step by step answer:
When haloalkanes with $\text{ }\beta -\text{ }$ hydrogen atoms are boiled with an alcoholic solution of potassium hydroxide, they undergo the elimination of hydrogen halide $\text{ }\left( \text{HX} \right)\text{ }$ resulting in the formation of alkenes. The general reaction of the dehydrohalogenation of the haloalkanes is as shown below,

These reactions are called the $\text{ }\beta -\text{ }$ elimination reactions because the hydrogen atom present at the $\text{ }\beta -\text{ }$ position of haloalkanes (i.e. at the carbon atom next to that which carries the halogen) is removed.
a) Ethyl bromide reaction with alc.$\text{ KOH }$:
The ethyl bromide reacted with the alcoholic potassium hydroxide $\text{ KOH }$. Here, bromine (halogen group) is at the $\text{ }\alpha \text{ }$ position and hydrogen is at the $\text{ }\beta \text{ }$ position. When boiled with
$\text{ KOH }$, hydrogen atom transfers its electron pair to the adjacent carbon-carbon bond, and bromide is removed from the molecule. This forms a double bond between the alpha and beta carbon atom and gives ethane as the product.

Thus, ethyl bromide on reaction with alcohol $\text{ KOH }$ gives ethene.
b) 2-chlorobutane reaction with alc.$\text{ KOH }$:
The 2-chlorobutane reacts with the alcoholic potassium hydroxide $\text{ KOH }$. Here, chlorine (halogen group) is at the $\text{ }\alpha \text{ }$ position and hydrogen is at the $\text{ }\beta \text{ }$ position. When boiled with
$\text{ KOH }$, hydrogen atom transfers its electron pair to the adjacent carbon-carbon bond, and bromide is removed from the molecule. This forms a double bond between the alpha and beta carbon atom and gives ethane as the product.
The 2-chlorobutane has the chloro group at the second carbon atom thus there are two $\text{ }\beta \text{ }$ positions. In the case of haloalkanes which has more than one $\text{ }\beta -\text{ }$ position can eliminate the hydrogen halide in two different ways, then that alkene will be preferred in which carbon atoms joined by the double bond are maximally alkylated. This rule is called the Saytzeff’s rule.

Thus, 2-chloro butane on reaction with alcohol $\text{ KOH }$ gives 2-butene as the major product. This is because the double-bonded carbons are linked to two methyl groups thus it is highly alkylated compared to 1-butene.
Note: Note that, if the substituted alkene formed during the elimination is capable of showing the cis-trans isomerism, then trans –alkene is always preferably formed as the major product because of its greater stability. For example here major product 2-butene exists in two isomers cis and trans and trans-isomer will be greater in amount. Thus we can say that $\text{ }\beta -\text{ }$ elimination of secondary halide results in three product major product (cis + trans isomers) and a minor product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




