
Activation energy of a reaction is:
a.) The energy released during the reaction.
b.) The energy evolves when activated complex is formed.
c.) Additional amount of energy needed by the reactants to overcome the potential barrier of reaction.
d.) The energy needed to form one mole of the product.
Answer
577.5k+ views
Hint: Activation energy can be calculated using various methods. It can be calculated using the Arrhenius equation and also when then two temperatures and the rate constant at both temperatures are known. The temperature should be converted to kelvin while calculating activation energy using the Arrhenius equation.
Complete step by step answer:
The activation energy refers to the minimum amount of energy which is required for the reaction to occur. If the amount of activation energy is less which does not meet the required need of activation energy for a reaction the process did not get successful which means that the reaction does not occur.
Activation energy is introduced by a scientist named Svante Arrhenius from Sweden. In the presence of the catalyst the activation energy gets lowered because the catalyst increases the rate of the reaction. Slower the chemical reaction higher will be the activation energy of the reaction. The release of heat also lowers the activation energy which is required by the reaction.
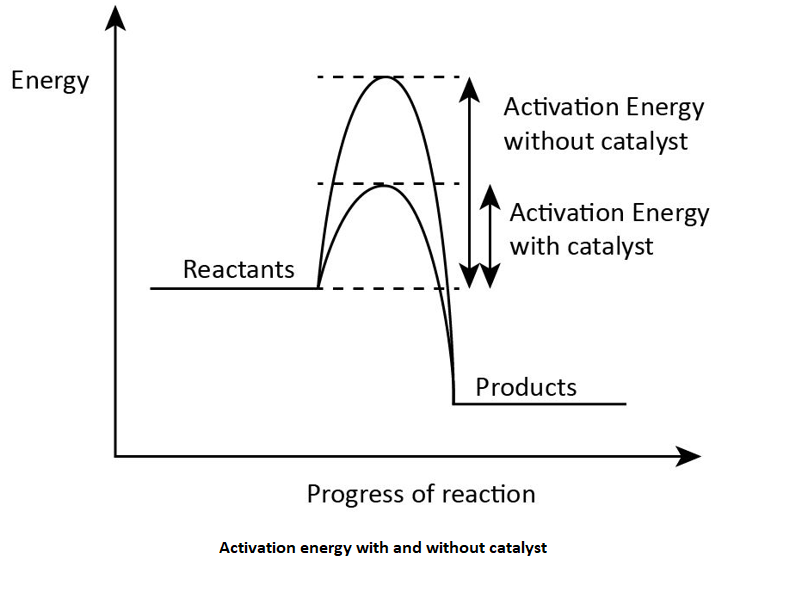
So, the correct answer is “Option C”.
Note: In terms of the transition state theory, the activation energy is the difference between the energy content of the atoms or the molecules in an activated or the transition state configuration. If the value of activation energy becomes zero there will be no effective collision and no product will be formed.
Complete step by step answer:
The activation energy refers to the minimum amount of energy which is required for the reaction to occur. If the amount of activation energy is less which does not meet the required need of activation energy for a reaction the process did not get successful which means that the reaction does not occur.
Activation energy is introduced by a scientist named Svante Arrhenius from Sweden. In the presence of the catalyst the activation energy gets lowered because the catalyst increases the rate of the reaction. Slower the chemical reaction higher will be the activation energy of the reaction. The release of heat also lowers the activation energy which is required by the reaction.

So, the correct answer is “Option C”.
Note: In terms of the transition state theory, the activation energy is the difference between the energy content of the atoms or the molecules in an activated or the transition state configuration. If the value of activation energy becomes zero there will be no effective collision and no product will be formed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




