
How do you add a hydroxyl group to an alkyl chain of a carboxylic acid?
Answer
527.4k+ views
Hint: We know that we must know that the functional groups are groups or specific substituent, functional groups are responsible for the chemical reactions characteristic of those molecules. Regardless of the size of the molecule the same functional group will undergo the same chemical reaction. Functional groups also give certain physical and chemical properties.
Complete answer:
We must know that for the specific chemical reactions of that molecule, the functional group is responsible for that molecule. To make a compound new and have desirable properties, the addition of some functional groups are given those properties and it is called functionalization. We must remember that a functional group attached at the end of the carbon chain (terminal position) is called the terminal functional group. If it is not attached at the end of the carbon chain then it is not an end functional group. In functional groups, carbonyl groups must be placed in the terminal position.
We can use the Hell-Volhard-Zelinski reaction to add it to the $ \alpha ~carbon. $ Recall that $ PB{{r}_{3~}} $ can be used to substitute a hydroxyl group for a $ Br. $ The enol tautomer of this new acyl bromide can attack a $ ~B{{r}_{2}} $ , then get hydrolyzed to return to a carboxylic acid, except with an $ \alpha - $ bromine.
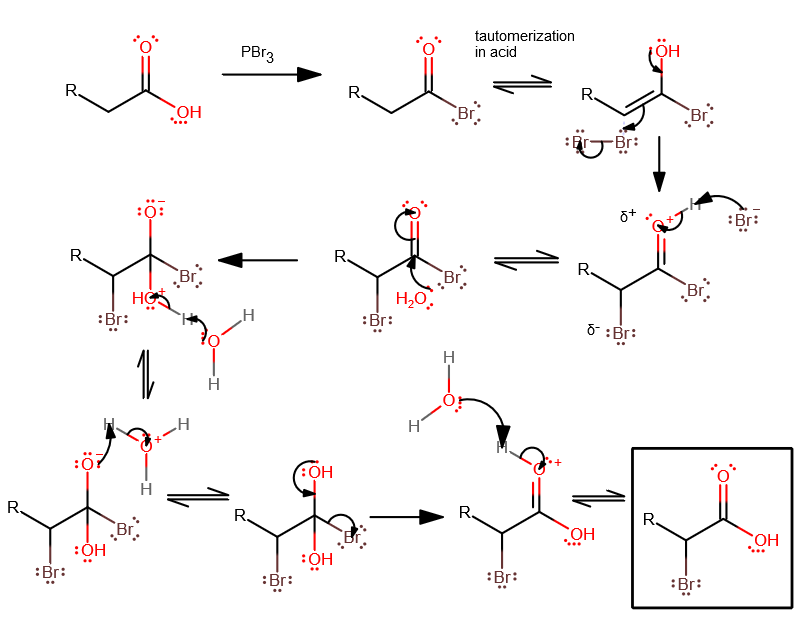
Note:
Remember that the functional groups have an important role in organic chemistry for controlling and directing the reactions, also the properties of the compound changes for different functional groups placed. Ketone contains the carbonyl at the inside of the compound but aldehydes are at the terminal positions. Both ketone and aldehyde are oxidized to give the corresponding carboxylic acids respectively.
Complete answer:
We must know that for the specific chemical reactions of that molecule, the functional group is responsible for that molecule. To make a compound new and have desirable properties, the addition of some functional groups are given those properties and it is called functionalization. We must remember that a functional group attached at the end of the carbon chain (terminal position) is called the terminal functional group. If it is not attached at the end of the carbon chain then it is not an end functional group. In functional groups, carbonyl groups must be placed in the terminal position.
We can use the Hell-Volhard-Zelinski reaction to add it to the $ \alpha ~carbon. $ Recall that $ PB{{r}_{3~}} $ can be used to substitute a hydroxyl group for a $ Br. $ The enol tautomer of this new acyl bromide can attack a $ ~B{{r}_{2}} $ , then get hydrolyzed to return to a carboxylic acid, except with an $ \alpha - $ bromine.

Note:
Remember that the functional groups have an important role in organic chemistry for controlling and directing the reactions, also the properties of the compound changes for different functional groups placed. Ketone contains the carbonyl at the inside of the compound but aldehydes are at the terminal positions. Both ketone and aldehyde are oxidized to give the corresponding carboxylic acids respectively.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




