
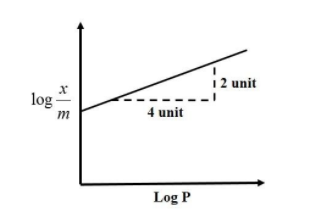
Adsorption of gas follows Freundlich adsorption isotherm. In the given plot, x is the mass of the gas adsorbed on the mass m of the adsorbent at pressure p. $\dfrac{{\text{x}}}{{\text{m}}}$ is proportional to:
(A) ${{\text{P}}^{\dfrac{{\text{1}}}{{\text{4}}}}}$
(B) ${{\text{P}}^{\text{2}}}$
(C) ${\text{P}}$
(D)${{\text{P}}^{\dfrac{{\text{1}}}{{\text{2}}}}}$

Answer
581.7k+ views
Hint: Knowledge on Freundlich adsorption isotherm is important to solve this question. The law states that the change in the amount of gas adsorbed by unit mass of a solid adsorbent is directly proportional to the change in the pressure, at a given temperature.
Formula used:
$\dfrac{{\text{x}}}{{\text{m}}}{\text{ = K}}{{\text{P}}^{\dfrac{{\text{1}}}{{\text{n}}}}}$
Where, x is the mass of the gas adsorbed on the mass m of the adsorbent at pressure P, K is a constant.
Complete step by step answer:
The Freundlich equation is expressed as:
$\dfrac{{\text{x}}}{{\text{m}}}{\text{ = K}}{{\text{P}}^{\dfrac{{\text{1}}}{{\text{n}}}}}$
Taking log base 10 both sides:
$ \Rightarrow \log \dfrac{{\text{x}}}{{\text{m}}} = \log E + \dfrac{1}{{\text{n}}}\log {\text{P}}$ Where (n>1)........... (1)
........... (2)
From the equation of a straight line we know that:
${\text{y}} = {\text{mx}} + {\text{c}}$,
Where the intercept of the equation, c = 0, ${\text{y = mx}}$
Therefore, the slope of the equation, ${\text{m = }}\dfrac{{\text{y}}}{{\text{x}}}$
Substituting the values, we get:
${\text{m}} = \dfrac{2}{4} = \dfrac{1}{2}$
As per equation (1), the slope of the equation is $\dfrac{1}{2}$=$\dfrac{1}{n}$
So, $\dfrac{{\text{x}}}{{\text{m}}} = {\text{K}}{{\text{P}}^{\dfrac{1}{2}}}$
Hence, option (D) is the correct answer.
Note:
The Freundlich adsorption isotherm is not valid at high pressures. This is because, experimentally, it was seen that the extent of gas adsorption varies directly with the pressure raised to the power $\dfrac{{\text{1}}}{{\text{n}}}$ till the saturation pressure is reached.
The quantity \[\dfrac{{\text{x}}}{{\text{m}}}\] is called the extent of absorption of a gas.
The extent of pressure increases with the pressure and becomes maximum at a pressure known as the “Equilibrium pressure” (P).
At the equilibrium pressure the amount of the gas adsorbed becomes equal to the amount of the gas desorbed, as adsorption and desorption take place simultaneously at this pressure.
Some observations include:
At low pressures: $\dfrac{{\text{x}}}{{\text{m}}}{\text{ = KP}}$
At high pressures: $\dfrac{{\text{x}}}{{\text{m}}}{\text{ = K}}{{\text{P}}^{\text{0}}}$
When the value of the pressure lies from 0 to 1, $\dfrac{{\text{x}}}{{\text{m}}} = {\text{K}}{{\text{P}}^{\dfrac{1}{{\text{n}}}}}$
Formula used:
$\dfrac{{\text{x}}}{{\text{m}}}{\text{ = K}}{{\text{P}}^{\dfrac{{\text{1}}}{{\text{n}}}}}$
Where, x is the mass of the gas adsorbed on the mass m of the adsorbent at pressure P, K is a constant.
Complete step by step answer:
The Freundlich equation is expressed as:
$\dfrac{{\text{x}}}{{\text{m}}}{\text{ = K}}{{\text{P}}^{\dfrac{{\text{1}}}{{\text{n}}}}}$
Taking log base 10 both sides:
$ \Rightarrow \log \dfrac{{\text{x}}}{{\text{m}}} = \log E + \dfrac{1}{{\text{n}}}\log {\text{P}}$ Where (n>1)........... (1)
........... (2)
From the equation of a straight line we know that:
${\text{y}} = {\text{mx}} + {\text{c}}$,
Where the intercept of the equation, c = 0, ${\text{y = mx}}$
Therefore, the slope of the equation, ${\text{m = }}\dfrac{{\text{y}}}{{\text{x}}}$
Substituting the values, we get:
${\text{m}} = \dfrac{2}{4} = \dfrac{1}{2}$
As per equation (1), the slope of the equation is $\dfrac{1}{2}$=$\dfrac{1}{n}$
So, $\dfrac{{\text{x}}}{{\text{m}}} = {\text{K}}{{\text{P}}^{\dfrac{1}{2}}}$
Hence, option (D) is the correct answer.
Note:
The Freundlich adsorption isotherm is not valid at high pressures. This is because, experimentally, it was seen that the extent of gas adsorption varies directly with the pressure raised to the power $\dfrac{{\text{1}}}{{\text{n}}}$ till the saturation pressure is reached.
The quantity \[\dfrac{{\text{x}}}{{\text{m}}}\] is called the extent of absorption of a gas.
The extent of pressure increases with the pressure and becomes maximum at a pressure known as the “Equilibrium pressure” (P).
At the equilibrium pressure the amount of the gas adsorbed becomes equal to the amount of the gas desorbed, as adsorption and desorption take place simultaneously at this pressure.
Some observations include:
At low pressures: $\dfrac{{\text{x}}}{{\text{m}}}{\text{ = KP}}$
At high pressures: $\dfrac{{\text{x}}}{{\text{m}}}{\text{ = K}}{{\text{P}}^{\text{0}}}$
When the value of the pressure lies from 0 to 1, $\dfrac{{\text{x}}}{{\text{m}}} = {\text{K}}{{\text{P}}^{\dfrac{1}{{\text{n}}}}}$
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




