
How many alcohols are structural isomers of ${{C}_{5}}{{H}_{11}}OH$?
(A) 6
(B) 7
(C) 8
(D) 9
Answer
523.9k+ views
Hint: Recollect the concept of isomerism. Find out what structural isomerism is. Start by writing the structure of pentanol. Then draw its structure in different forms by changing the substituents and placing alcohol groups in different positions. Count all the structures to get the answer.
Complete step by step solution:
- Structural isomers are compounds having the same molecular formula and different structural formulae. This phenomenon is known as structural isomerism.
- The structure of pentanol, ${{C}_{5}}{{H}_{11}}OH$ is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH$.
- Now, the question says the number of structural isomers of alcohol. So, the functional group needs to be alcohol only in all the isomers.
- Let’s draw all the possible structural isomers of pentanol.
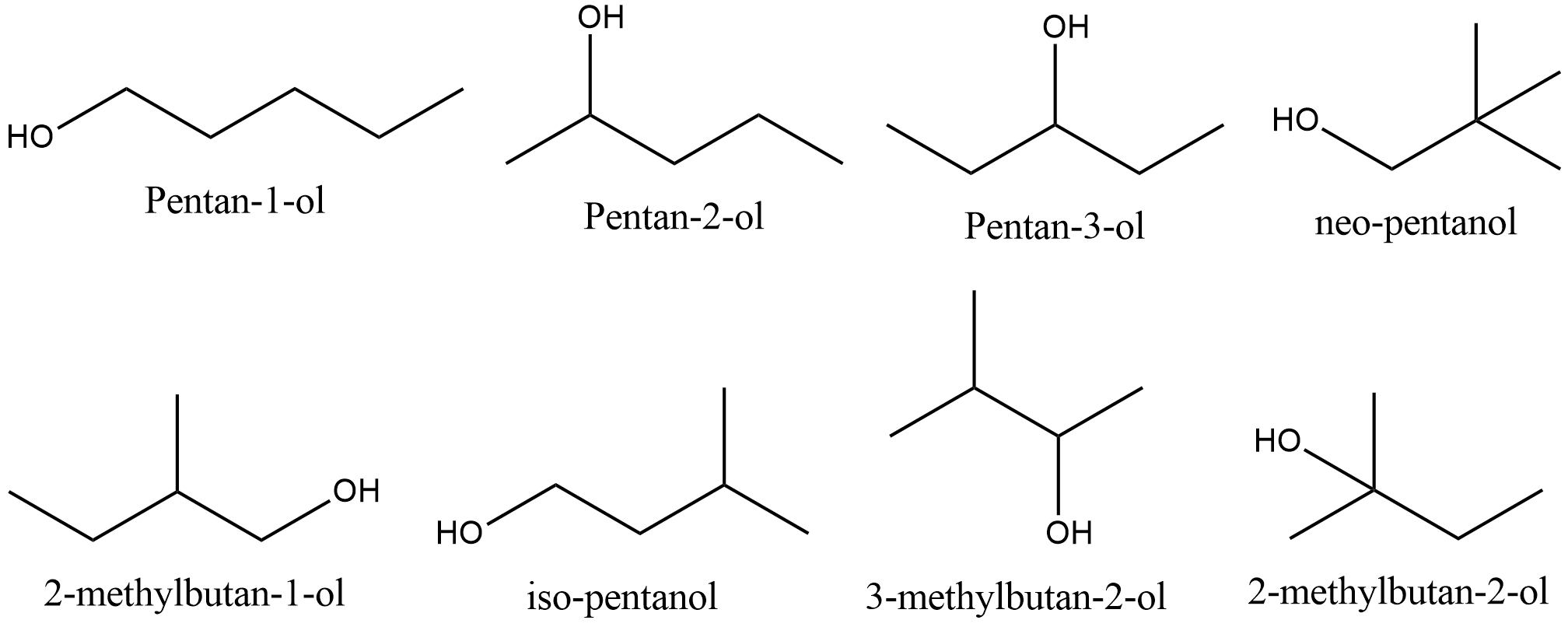
- There are eight structural isomeric alcohols of pentanol.
- They are as follows:
- Pentan-1-ol
- Pentan-2-ol
- Pentan-3-ol
- neo-pentanol
- 2-methylbutan-1-ol
- iso-pentanol
- 3-methylbutan-2-ol
- 2-methylbutan-2-ol
- Therefore, alcoholic structural isomers having the molecular formula, ${{C}_{5}}{{H}_{11}}OH$ are eight.
Therefore, the correct answer is option C.
Note: Remember structural isomers are the compounds having the same molecular formula but different structural formulae. For alcohols, ethers are also structural isomers. So, ${{C}_{5}}{{H}_{11}}OH$ also has more structural isomers. Depending on the way of structural arrangement of atoms, more isomers of ${{C}_{5}}{{H}_{11}}OH$ are ethyl propyl ether, butyl methyl ether, ethyl isopropyl ether, tert-butyl methyl ether, iso-butyl methyl ether, etc.
Complete step by step solution:
- Structural isomers are compounds having the same molecular formula and different structural formulae. This phenomenon is known as structural isomerism.
- The structure of pentanol, ${{C}_{5}}{{H}_{11}}OH$ is $C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}OH$.
- Now, the question says the number of structural isomers of alcohol. So, the functional group needs to be alcohol only in all the isomers.
- Let’s draw all the possible structural isomers of pentanol.

- There are eight structural isomeric alcohols of pentanol.
- They are as follows:
- Pentan-1-ol
- Pentan-2-ol
- Pentan-3-ol
- neo-pentanol
- 2-methylbutan-1-ol
- iso-pentanol
- 3-methylbutan-2-ol
- 2-methylbutan-2-ol
- Therefore, alcoholic structural isomers having the molecular formula, ${{C}_{5}}{{H}_{11}}OH$ are eight.
Therefore, the correct answer is option C.
Note: Remember structural isomers are the compounds having the same molecular formula but different structural formulae. For alcohols, ethers are also structural isomers. So, ${{C}_{5}}{{H}_{11}}OH$ also has more structural isomers. Depending on the way of structural arrangement of atoms, more isomers of ${{C}_{5}}{{H}_{11}}OH$ are ethyl propyl ether, butyl methyl ether, ethyl isopropyl ether, tert-butyl methyl ether, iso-butyl methyl ether, etc.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




