
Alicyclic compounds are:
a.) Aromatic compounds
b.) Aliphatic compounds
c.) Heterocyclic compounds
d.) Aliphatic cyclic compounds
Answer
596.7k+ views
Hint: Non aromatic cyclic compounds are called alicyclic compounds. Non aromatic compounds are called aliphatic. It may be unicyclic or multicyclic.
Complete step by step solution:
The compounds which have one or more all-carbon rings, either saturated or unsaturated but do not have aromatic character are called alicyclic compounds. In other words, alicyclic compounds are aliphatic cyclic compounds. The bonds between pairs of adjacent atoms may all be of the type designated single bonds (involving two electrons), or some of them may be double or triple bonds (with four or six electrons, respectively); six-membered rings for which a system of alternating single and double bonds may be envisioned. Alicyclic compounds may have one or more aliphatic side chains attached.
The simplest alicyclic compounds are the monocyclic cycloalkanes. Monocyclic cycloalkanes include: cyclopropane, cyclobutene, cyclopentane, cyclohexane, cycloheptane, cyclooctane, etc.
Bicyclic alkanes include: bicyclo undecane, decalin, etc.
Polycyclic alkanes include cubane, basketane and tetrahedrane.
The smallest alicyclic compound is cyclopropane. The mode of ring-closing in the formation of many alicyclic compounds can be predicted by Baldwin's rules.
Those alicyclic compounds in which the ring contains three or four carbon atoms are less stable than the compounds having larger rings, because the angles formed by adjacent covalent bonds are smaller than is necessary for maximum effectiveness. In the larger rings all the bond angles have the preferred value (about 109.5°); consequently, the atoms in the ring do not lie in one plane.
Some structures of alicyclics are:
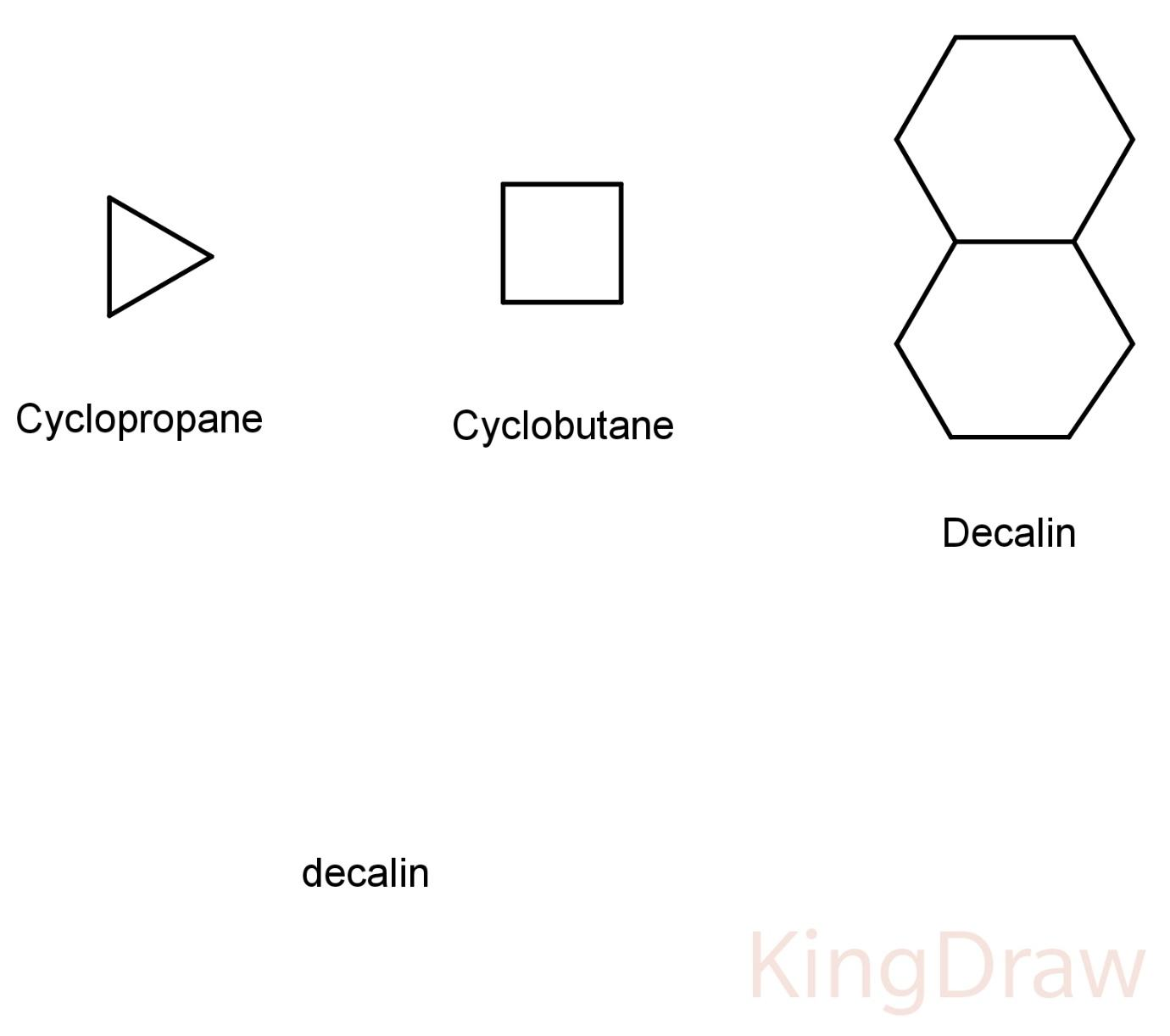
Hence, the correct answer is (D) aliphatic cyclic compounds.
Note: A student must know that homocyclic organic compounds are classified into aromatic and alicyclic compounds. Remember that the alicyclic compounds may have one or more aliphatic side chains attached.
Complete step by step solution:
The compounds which have one or more all-carbon rings, either saturated or unsaturated but do not have aromatic character are called alicyclic compounds. In other words, alicyclic compounds are aliphatic cyclic compounds. The bonds between pairs of adjacent atoms may all be of the type designated single bonds (involving two electrons), or some of them may be double or triple bonds (with four or six electrons, respectively); six-membered rings for which a system of alternating single and double bonds may be envisioned. Alicyclic compounds may have one or more aliphatic side chains attached.
The simplest alicyclic compounds are the monocyclic cycloalkanes. Monocyclic cycloalkanes include: cyclopropane, cyclobutene, cyclopentane, cyclohexane, cycloheptane, cyclooctane, etc.
Bicyclic alkanes include: bicyclo undecane, decalin, etc.
Polycyclic alkanes include cubane, basketane and tetrahedrane.
The smallest alicyclic compound is cyclopropane. The mode of ring-closing in the formation of many alicyclic compounds can be predicted by Baldwin's rules.
Those alicyclic compounds in which the ring contains three or four carbon atoms are less stable than the compounds having larger rings, because the angles formed by adjacent covalent bonds are smaller than is necessary for maximum effectiveness. In the larger rings all the bond angles have the preferred value (about 109.5°); consequently, the atoms in the ring do not lie in one plane.
Some structures of alicyclics are:

Hence, the correct answer is (D) aliphatic cyclic compounds.
Note: A student must know that homocyclic organic compounds are classified into aromatic and alicyclic compounds. Remember that the alicyclic compounds may have one or more aliphatic side chains attached.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




