
Alkaline $KMn{{O}_{4}}$ (Bayer’s reagent) can be used to test unsaturation in (A). in this case
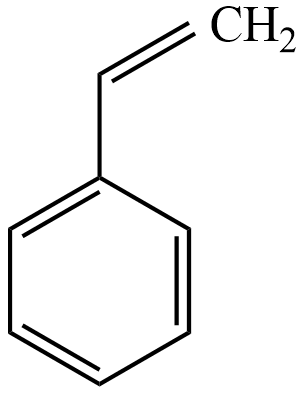
(A) unsaturation in side-chain affected
(B) unsaturation in benzene nucleus is affected
(C) unsaturation in both is affected
(D) Bayer’s reagent cannot be used

Answer
581.7k+ views
Hint: The alkynes and alkenes are coming under unsaturated hydrocarbons. In organic chemistry, there are many tests to identify the presence of unsaturated in hydrocarbons. The alkaline potassium permanganate test (Baeyer’s test) and bromine test are the methods for detecting the unsaturation in an organic compound.
Complete step by step answer:
Baeyer’ reagent, which is an alkaline solution of cold potassium permanganate ($KMn{{O}_{4}}$ ). In the qualitative organic analysis, the alkaline potassium permanganate test is used to identify the presence of unsaturation compounds like alkyne and alkene.
When this Baeyer’s reagent interacts with unsaturated compounds changing its pinkish-purple color to brown, because of the alkenes are oxidized to 1,2-diol and the permanganate is reduced to manganese dioxide( $Mn{{O}_{2}}$ ). In this reaction, potassium permanganate acts as an oxidizing agent.
The test does not work for alkanes and aromatic compounds, and hence, this Baeyer’s test is used to distinguish them from alkenes and alkynes.
So, Alkaline $KMn{{O}_{4}}$ (Baeyer’s reagent) can be used to test unsaturation in the given compound, unsaturation in side-chain only affected, and this test not affected the benzene ring in the compound.
So, the correct answer is “Option A”.
Note: Baeyer’s reagent has more applications in medical purpose, in water treatment, synthesis of organic compounds, analytical methods, fruit preservation, survival kits, and fire service. Potassium permanganate dissolves in water to give intense pink or purple solutions and leaves purplish-black crystals on evaporation.
Complete step by step answer:
Baeyer’ reagent, which is an alkaline solution of cold potassium permanganate ($KMn{{O}_{4}}$ ). In the qualitative organic analysis, the alkaline potassium permanganate test is used to identify the presence of unsaturation compounds like alkyne and alkene.
When this Baeyer’s reagent interacts with unsaturated compounds changing its pinkish-purple color to brown, because of the alkenes are oxidized to 1,2-diol and the permanganate is reduced to manganese dioxide( $Mn{{O}_{2}}$ ). In this reaction, potassium permanganate acts as an oxidizing agent.
The test does not work for alkanes and aromatic compounds, and hence, this Baeyer’s test is used to distinguish them from alkenes and alkynes.
So, Alkaline $KMn{{O}_{4}}$ (Baeyer’s reagent) can be used to test unsaturation in the given compound, unsaturation in side-chain only affected, and this test not affected the benzene ring in the compound.
So, the correct answer is “Option A”.
Note: Baeyer’s reagent has more applications in medical purpose, in water treatment, synthesis of organic compounds, analytical methods, fruit preservation, survival kits, and fire service. Potassium permanganate dissolves in water to give intense pink or purple solutions and leaves purplish-black crystals on evaporation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




