
When an alkene is mixed with bromine water:
A. The bromine water changes from orange to colourless
B. The bromine water stays orange
C. The bromine water changes from colourless to orange
D. The bromine water change from brown to colourless
Answer
592.8k+ views
Hint: Alkenes react with a solution of bromine in an organic solvent like tetrachloromethane. The double bond breaks out and bromine atoms get added to each carbon. The reaction is marked by the change in the colour of the solution.
Complete answer:
The reaction between alkene and bromine water is an example of electrophilic addition. Bromine has ionic properties: $\left( \text{B}{{\text{r}}^{-}} \right)$. Due to this property the bromine induces charges in pi-bond also. Let us see the mechanism of this reaction to understand this reaction completely:
Step (1)- The bromine-bromine bond breaks to form bromonium ion. Bromine atoms induce the charge to double bond and attack the carbon atoms. The structure formed is triangle-shaped.
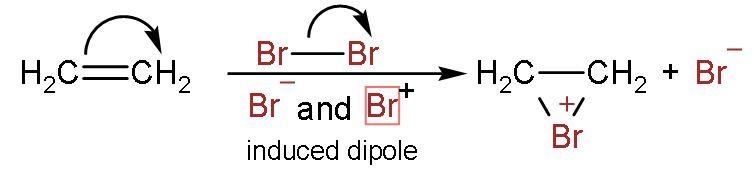
Step (2)- The bromonium ion is attacked from the back side due to hindrance faced on the same side by bromide ion.
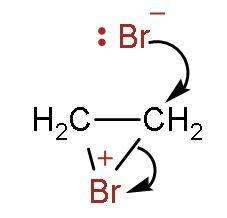
Step (3)- It attaches to other double bonded carbon, and thus the product is formed. The two bromine atoms are attached to both carbon atoms containing double bonds.
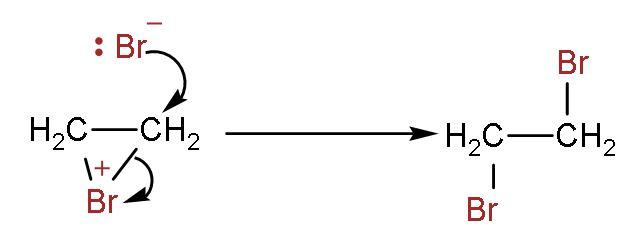
As a result, the bromine water loses its original red-brown or orange colour to give a colourless liquid. So, the correct answer is “Option A”.
Additional Information: This decolourisation of bromine is used as a test for presence of carbon-carbon double bond in the hydrocarbons. This technique is used to distinguish between alkenes and alkanes.
Note: Not all compounds having double bonds show this reaction of addition of bromines. Such compounds include benzene. Benzene has three double bonds in it, but still does not leave bromine water colourless. This reaction does not occur because benzene does not undergo electrophilic addition reaction on addition of bromines, the aromaticity of benzene would have been lost. The 6 pi electrons ($6\pi {{\text{e}}^{-}}$) would have become 4 pi electrons ($4\pi {{\text{e}}^{-}}$). As, according to Huckel’s Rule, compound having conjugated $6\pi {{\text{e}}^{-}}$ within the ring is aromatic.
Complete answer:
The reaction between alkene and bromine water is an example of electrophilic addition. Bromine has ionic properties: $\left( \text{B}{{\text{r}}^{-}} \right)$. Due to this property the bromine induces charges in pi-bond also. Let us see the mechanism of this reaction to understand this reaction completely:
Step (1)- The bromine-bromine bond breaks to form bromonium ion. Bromine atoms induce the charge to double bond and attack the carbon atoms. The structure formed is triangle-shaped.

Step (2)- The bromonium ion is attacked from the back side due to hindrance faced on the same side by bromide ion.

Step (3)- It attaches to other double bonded carbon, and thus the product is formed. The two bromine atoms are attached to both carbon atoms containing double bonds.

As a result, the bromine water loses its original red-brown or orange colour to give a colourless liquid. So, the correct answer is “Option A”.
Additional Information: This decolourisation of bromine is used as a test for presence of carbon-carbon double bond in the hydrocarbons. This technique is used to distinguish between alkenes and alkanes.
Note: Not all compounds having double bonds show this reaction of addition of bromines. Such compounds include benzene. Benzene has three double bonds in it, but still does not leave bromine water colourless. This reaction does not occur because benzene does not undergo electrophilic addition reaction on addition of bromines, the aromaticity of benzene would have been lost. The 6 pi electrons ($6\pi {{\text{e}}^{-}}$) would have become 4 pi electrons ($4\pi {{\text{e}}^{-}}$). As, according to Huckel’s Rule, compound having conjugated $6\pi {{\text{e}}^{-}}$ within the ring is aromatic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




