
$\alpha $ And $\beta $ forms of Sulphur, both are stable at:
(A) ${369^ \circ }C$
(B) 369K
(C) ${36^ \circ }C$
(D) ${69^ \circ }C$
Answer
589.5k+ views
Hint: Sulphur is a chemical element with the symbol S and atomic number 16. It is a non-metal and is obtained as a byproduct after the production of natural gas. It is bright yellow in color. The two most important allotropes of Sulphur are yellow rhombic Sulphur ($\alpha $ Sulphur) and monoclinic ($\beta $ Sulphur).
Complete step by step answer:
Sulphur is the tenth most common element by mass in the universe and the fifth most common on the Earth. It is found in group 16 in the periodic table.
Further, it consists of two main allotropes namely yellow rhombic Sulphur ($\alpha $ Sulphur) and monoclinic ($\beta $ Sulphur). The allotropes of Sulphur are inter-convertible i.e. rhombic Sulphur when heated above $369K$ gives monoclinic Sulphur.
Now, rhombic Sulphur is crystalline in nature and has an octahedral shape. It cannot be dissolved in water but can be dissolved in benzene, ether and alcohol whereas when we take a dish and melt the rhombic sulphur, we obtain monoclinic sulphur after cooling it.
The most interesting fact is that both the allotropes of sulphur are stable at a temperature of $369K$ and this temperature is known as transition temperature. In other words, we can say that $\alpha $ Sulphur is stable below 369K and it becomes $\beta $ Sulphur above that temperature.
Their structures are as shown:
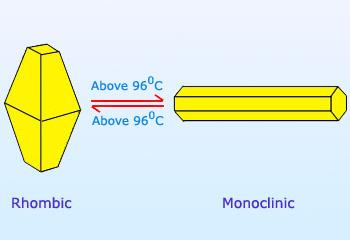
Hence, option B is correct.
Note: Sulphur plays a vital role in our ecosystem by affecting the growth of plants. This has led to the development of many sulphur containing fertilizers. Moreover, it is used in many bleaching agents and also in the manufacturing of carbon disulphide which in turn is used in skin ointments and other such products.
Complete step by step answer:
Sulphur is the tenth most common element by mass in the universe and the fifth most common on the Earth. It is found in group 16 in the periodic table.
Further, it consists of two main allotropes namely yellow rhombic Sulphur ($\alpha $ Sulphur) and monoclinic ($\beta $ Sulphur). The allotropes of Sulphur are inter-convertible i.e. rhombic Sulphur when heated above $369K$ gives monoclinic Sulphur.
Now, rhombic Sulphur is crystalline in nature and has an octahedral shape. It cannot be dissolved in water but can be dissolved in benzene, ether and alcohol whereas when we take a dish and melt the rhombic sulphur, we obtain monoclinic sulphur after cooling it.
The most interesting fact is that both the allotropes of sulphur are stable at a temperature of $369K$ and this temperature is known as transition temperature. In other words, we can say that $\alpha $ Sulphur is stable below 369K and it becomes $\beta $ Sulphur above that temperature.
Their structures are as shown:

Hence, option B is correct.
Note: Sulphur plays a vital role in our ecosystem by affecting the growth of plants. This has led to the development of many sulphur containing fertilizers. Moreover, it is used in many bleaching agents and also in the manufacturing of carbon disulphide which in turn is used in skin ointments and other such products.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




