
\[\alpha \] -D-fructofuranose is an aldopentose.
A) True
B) False
Answer
582.9k+ views
Hint: We know that D-fructofuranose is a cyclic structure of furanose and it contains one ketonic group and six-carbon atom including the ketonic group. Structures containing the aldehyde group come under aldopentoses.
Complete step by step answer:
Let us see the structure of D-fructose:
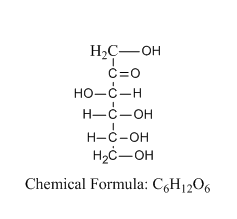
From the structure, we can tell that the structure consists of six carbon atoms and a ketone group at position number 2.
Now let us see the structure of \[\alpha \] -D-fructofuranose:
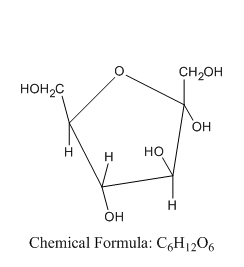
From this, we can say that the \[\alpha \] -D-fructofuranose comes under the group of hex ketose and not aldopentose.
Hence the statement is a false statement.
Therefore, we can conclude that the correct answer to this question is option B.
Additional information:
-We know that Fructose is a simple ketone monosaccharide found in plants where it is often bonded to glucose to form the disaccharide sucrose. We also know that fructose is a 6-carbon polyhydroxy ketone and crystalline fructose adopts a cyclic six-membered structure owing to the stability of its hemiketal and internal hydrogen-bonding and this structure is -generally called as D-fructofuranose.
We must know that fructose is highly soluble in water than any other natural form of sugars, as well as other sugar alcohols.
-Hence it is very difficult to crystallize fructose from the aqueous solution.
-Fructose consists of two forms of hemiketal isomers.
-We know that the fructose is found in honey, berries and in maximum number of fruits.
-It is observed that in water solution, fructose exists as a mixture of \[70\% \] fructopyranose and about \[22\% \] fructofuranose, and also small amounts of three other forms which also includes the acyclic structure.
Note:
In this type of question we have to focus on the structure of the \[\alpha \] -D-fructofuranose. Only by seeing the structure, we can find that as it does not contain an aldehyde group it cannot come under the category of aldopentose.
Complete step by step answer:
Let us see the structure of D-fructose:

From the structure, we can tell that the structure consists of six carbon atoms and a ketone group at position number 2.
Now let us see the structure of \[\alpha \] -D-fructofuranose:

From this, we can say that the \[\alpha \] -D-fructofuranose comes under the group of hex ketose and not aldopentose.
Hence the statement is a false statement.
Therefore, we can conclude that the correct answer to this question is option B.
Additional information:
-We know that Fructose is a simple ketone monosaccharide found in plants where it is often bonded to glucose to form the disaccharide sucrose. We also know that fructose is a 6-carbon polyhydroxy ketone and crystalline fructose adopts a cyclic six-membered structure owing to the stability of its hemiketal and internal hydrogen-bonding and this structure is -generally called as D-fructofuranose.
We must know that fructose is highly soluble in water than any other natural form of sugars, as well as other sugar alcohols.
-Hence it is very difficult to crystallize fructose from the aqueous solution.
-Fructose consists of two forms of hemiketal isomers.
-We know that the fructose is found in honey, berries and in maximum number of fruits.
-It is observed that in water solution, fructose exists as a mixture of \[70\% \] fructopyranose and about \[22\% \] fructofuranose, and also small amounts of three other forms which also includes the acyclic structure.
Note:
In this type of question we have to focus on the structure of the \[\alpha \] -D-fructofuranose. Only by seeing the structure, we can find that as it does not contain an aldehyde group it cannot come under the category of aldopentose.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




