
Aluminium occurs as a compound whereas gold is found in free State. Why?
Answer
603.3k+ views
Hint: To solve this question, we should know that very few metals are present in the universe that can resist natural weathering processes like oxidation, which is why generally only the less reactive metals such as native metals.
Step by step answer:
We should first know about native metals. A native metal is any metal that is found pure in its metallic form in nature. Only few metals are there which can resist natural weathering processes like oxidation, which is why generally only the less reactive metals such as gold and platinum are found as native metals.
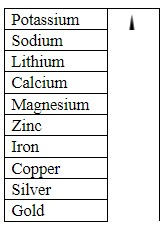
The above table shows reactivity of different metals. A good way to remember the order of a reactivity series of metals is to use the first letter of each one to make up a silly sentence. For example: People Say Little Children Make a Zebra Ill Constantly Sniffing Giraffes. As we can see that gold is placed at last in order of reactivity series. So, this states that gold will exist in a free state in the universe.
We should note that aluminium can be difficult to place in the correct position in the reactivity series during these experiments. This is because its protective aluminium oxide layer makes it appear to be less reactive than it really is. When this layer is removed, the observations are more reliable. We should know that gold is very less reactive. On the contrary the aluminium metal is highly reactive. Therefore aluminium reacts with other elements and compounds more than gold therefore, Aluminium occurs as a compound whereas gold is found in the free state.
Note: We should note that a more reactive metal will displace a less reactive metal from a compound. Let us remember the thermite reaction; it is a good example of this. It is used to produce white hot molten (liquid) iron in remote locations for welding. A lot of heat is needed to start the reaction, but then it releases an incredible amount of heat, enough to melt the iron.
\[Aluminium+iron\text{ }\left( III \right)\text{ }oxide\to iron+aluminium\text{ }oxide\]
\[2Al\text{ }+\text{ }F{{e}_{2}}{{O}_{3}}~\to \text{ }2Fe\text{ }+\text{ }A{{l}_{2}}{{O}_{3}}\]
Because aluminium is more reactive than iron, it displaces iron from iron (III) oxide. The aluminium removes oxygen from the iron (III) oxide. In this iron is reduced and aluminium is oxidised.
Step by step answer:
We should first know about native metals. A native metal is any metal that is found pure in its metallic form in nature. Only few metals are there which can resist natural weathering processes like oxidation, which is why generally only the less reactive metals such as gold and platinum are found as native metals.

The above table shows reactivity of different metals. A good way to remember the order of a reactivity series of metals is to use the first letter of each one to make up a silly sentence. For example: People Say Little Children Make a Zebra Ill Constantly Sniffing Giraffes. As we can see that gold is placed at last in order of reactivity series. So, this states that gold will exist in a free state in the universe.
We should note that aluminium can be difficult to place in the correct position in the reactivity series during these experiments. This is because its protective aluminium oxide layer makes it appear to be less reactive than it really is. When this layer is removed, the observations are more reliable. We should know that gold is very less reactive. On the contrary the aluminium metal is highly reactive. Therefore aluminium reacts with other elements and compounds more than gold therefore, Aluminium occurs as a compound whereas gold is found in the free state.
Note: We should note that a more reactive metal will displace a less reactive metal from a compound. Let us remember the thermite reaction; it is a good example of this. It is used to produce white hot molten (liquid) iron in remote locations for welding. A lot of heat is needed to start the reaction, but then it releases an incredible amount of heat, enough to melt the iron.
\[Aluminium+iron\text{ }\left( III \right)\text{ }oxide\to iron+aluminium\text{ }oxide\]
\[2Al\text{ }+\text{ }F{{e}_{2}}{{O}_{3}}~\to \text{ }2Fe\text{ }+\text{ }A{{l}_{2}}{{O}_{3}}\]
Because aluminium is more reactive than iron, it displaces iron from iron (III) oxide. The aluminium removes oxygen from the iron (III) oxide. In this iron is reduced and aluminium is oxidised.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




