
How is ammonia represented by an electron dot diagram?
Answer
555k+ views
Hint: An electron dot diagram is also known as the Lewis diagram after it was discovered by Lewis. It is a representation of the valence electrons that are involved in the bonding/non-bonding of an atom. The number of dots represented the valence electrons present in the given atom
Complete step by step answer:
It is known that a bond is formed by two electrons. Ionic, covalent, and coordinate covalent are the types of bonds present in the compound.
In an ammonia structure, one nitrogen is covalently bonded to three hydrogen atoms.
The outer electronic configuration of nitrogen can be written as,
\[N = 2{s^2}2{p^3}\]
It is known that the valency of nitrogen is 3 and hence, it can form three bonds with other atoms. Nitrogen also contains one lone pair of electrons. The lone pair of electrons is nothing but the electrons which are not involved in bonding.
The electron dot diagram of ammonia can be drawn as,
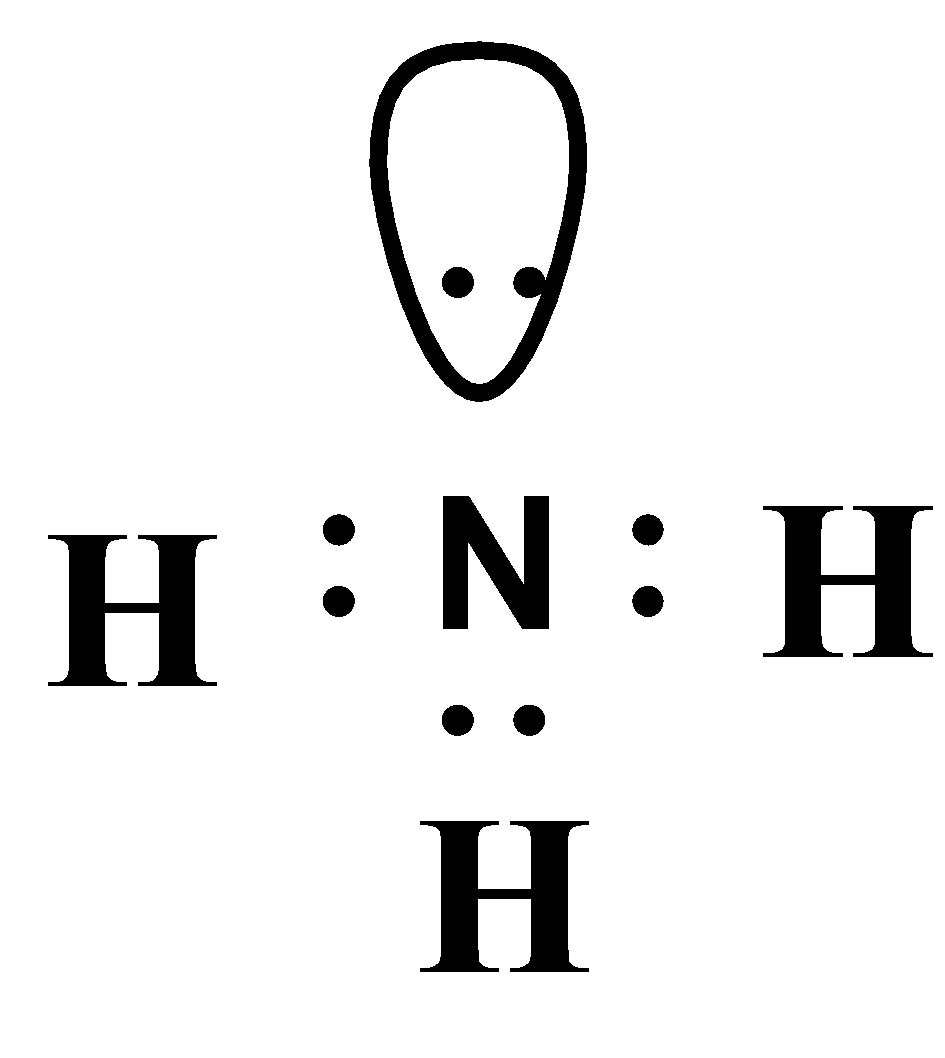
The bonding involved in ammonia is purely covalent. Since, nitrogen and hydrogen share their electrons and thus, they attained a stable octet (8 electrons) configuration.
Note: Ionic bond involves the transfer of electrons from one atom to another. The compound which is formed by an ionic bond produces ions. A well-known example of an ionic bond is sodium chloride. A covalent bond involves the sharing of electrons between the atoms. The compound which is formed by covalent bond has a stable octet configuration and also, it won't split as ions. The well-known example of a covalent bond is oxygen molecule, ammonia, etc. A coordinate covalent bond involves the complete sharing of a pair of electrons by one atom. The well-known example is \[N{H_3} \to B{H_3}\]
Complete step by step answer:
It is known that a bond is formed by two electrons. Ionic, covalent, and coordinate covalent are the types of bonds present in the compound.
In an ammonia structure, one nitrogen is covalently bonded to three hydrogen atoms.
The outer electronic configuration of nitrogen can be written as,
\[N = 2{s^2}2{p^3}\]
It is known that the valency of nitrogen is 3 and hence, it can form three bonds with other atoms. Nitrogen also contains one lone pair of electrons. The lone pair of electrons is nothing but the electrons which are not involved in bonding.
The electron dot diagram of ammonia can be drawn as,

The bonding involved in ammonia is purely covalent. Since, nitrogen and hydrogen share their electrons and thus, they attained a stable octet (8 electrons) configuration.
Note: Ionic bond involves the transfer of electrons from one atom to another. The compound which is formed by an ionic bond produces ions. A well-known example of an ionic bond is sodium chloride. A covalent bond involves the sharing of electrons between the atoms. The compound which is formed by covalent bond has a stable octet configuration and also, it won't split as ions. The well-known example of a covalent bond is oxygen molecule, ammonia, etc. A coordinate covalent bond involves the complete sharing of a pair of electrons by one atom. The well-known example is \[N{H_3} \to B{H_3}\]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




