
Among $Ca{H_2},N{H_3},NaH$ and ${B_2}{H_6}$, which are covalent hydrides?
A) $N{H_3}$ and ${B_2}{H_6}$
B) $NaH$ and $Ca{H_2}$
C) $NaH$ and $N{H_3}$
D) $Ca{H_2}$ and ${B_2}{H_6}$
Answer
565.5k+ views
Hint:A compound will be covalent or ionic is based on the oxidation state of the central atom present in the compound if it exceeds a predefined value the compound is covalent else it is ionic in nature. All the metal hydrides of group one of periodic table are ionic in nature due to high electro-positive nature of alkali metals.
Complete step by step answer:
As we know that sodium $\left( {Na} \right)$ belongs to group one of the periodic table and is an alkali metal which is highly electropositive in nature hence it will always form ionic compound thus a hydride of sodium will also be ionic in nature hence $NaH$ is ionic in nature
Also if we find the oxidation state of sodium is $ + 1$ which is very low that way also it is ionic in nature
Calcium $\left( {Ca} \right)$ belongs to group two of the periodic table and is an alkaline earth metal even though it is less electropositive than sodium but still has tendency to form only ionic bond which means $Ca{H_2}$ is also Ionic in nature
Just like sodium, calcium also exist in a very low oxidation state of $ + 2$ in $Ca{H_2}$ and that also makes it an Ionic compound
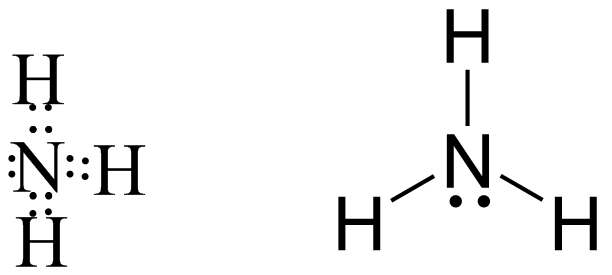
Now coming to $N{H_3}$ we can see in the following image that the central atom is nitrogen which is highly electronegative in nature hence it generally forms covalent bond also there is not much net electronegativity difference between $N$ and $H$ that also confirms its covalent nature
Finally coming to ${B_2}{H_6}$ in which central atom is Boron which is belongs to group $13$ of periodic table shows completely different behavior from the other group member because of its anomalous nature
Forms a three centered two electron covalent bond in ${B_2}{H_6}$
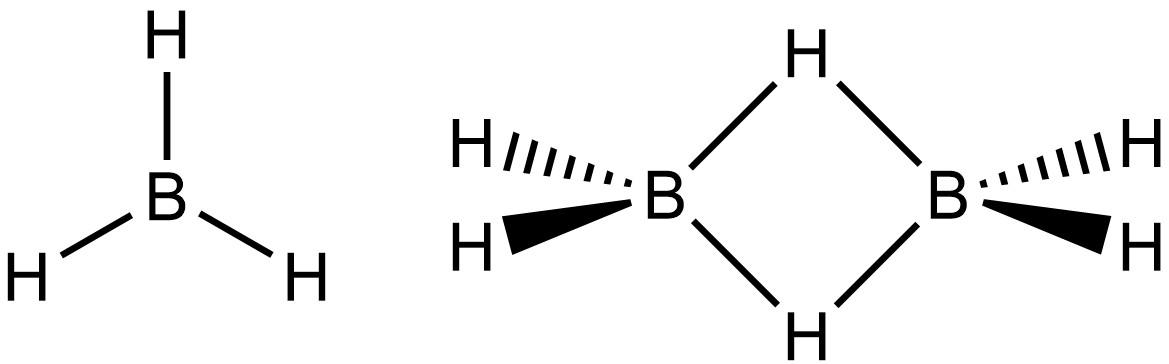
Borane Diborane
Thus from the above four hydrides only two that are ${B_2}{H_6}$ and $N{H_3}$ are covalent in nature
Making option ‘A’ as the correct option.
Note:
The special type of three centered two electron covalent bond in ${B_2}{H_6}$ is known as Banana bond and is asked in various competitive exams so do remember that Diborane (${B_2}{H_6}$) is an example of a compound forming banana bond.
Complete step by step answer:
As we know that sodium $\left( {Na} \right)$ belongs to group one of the periodic table and is an alkali metal which is highly electropositive in nature hence it will always form ionic compound thus a hydride of sodium will also be ionic in nature hence $NaH$ is ionic in nature
Also if we find the oxidation state of sodium is $ + 1$ which is very low that way also it is ionic in nature
Calcium $\left( {Ca} \right)$ belongs to group two of the periodic table and is an alkaline earth metal even though it is less electropositive than sodium but still has tendency to form only ionic bond which means $Ca{H_2}$ is also Ionic in nature
Just like sodium, calcium also exist in a very low oxidation state of $ + 2$ in $Ca{H_2}$ and that also makes it an Ionic compound
Now coming to $N{H_3}$ we can see in the following image that the central atom is nitrogen which is highly electronegative in nature hence it generally forms covalent bond also there is not much net electronegativity difference between $N$ and $H$ that also confirms its covalent nature

Finally coming to ${B_2}{H_6}$ in which central atom is Boron which is belongs to group $13$ of periodic table shows completely different behavior from the other group member because of its anomalous nature
Forms a three centered two electron covalent bond in ${B_2}{H_6}$

Borane Diborane
Thus from the above four hydrides only two that are ${B_2}{H_6}$ and $N{H_3}$ are covalent in nature
Making option ‘A’ as the correct option.
Note:
The special type of three centered two electron covalent bond in ${B_2}{H_6}$ is known as Banana bond and is asked in various competitive exams so do remember that Diborane (${B_2}{H_6}$) is an example of a compound forming banana bond.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




