
Among the following nuclides, the highest binding energy per nucleon is found for:
(A) ${}_1^3H$
(B) ${}_8^{16}O$
(C) ${}_{26}^{56}Fe$
(D) ${}_{92}^{235}U$
Answer
577.2k+ views
Hint: The average energy per nucleon which we will need to separate a nucleus into its constituent nucleons is the binding energy per nucleon. The binding energy per nucleon is low for both the light and heavy nuclei.
Complete step by step answer:
-First of all let us see what binding energy is.
Binding energy is the energy released when some protons and neutrons are brought together to form a nucleus of some specific charge and mass. It is denoted as ${E_b}$. If we need to separate the protons and neutrons of a nucleus then we will need to provide this binding energy to the particles.
-Now let us talk about binding energy per nucleon.
Binding energy per nucleon is defined as the ratio of the binding energy of the nucleus to the number of nucleons present in the nucleus. It is mathematically written as:
${E_{bn}} = \dfrac{{{E_b}}}{A}$
Where, ${E_b}$ = binding energy; and A = number of nucleons in it.
-There is a graph which shows us the binding energy per nucleon for various nuclei. A simplified graph is shown below:
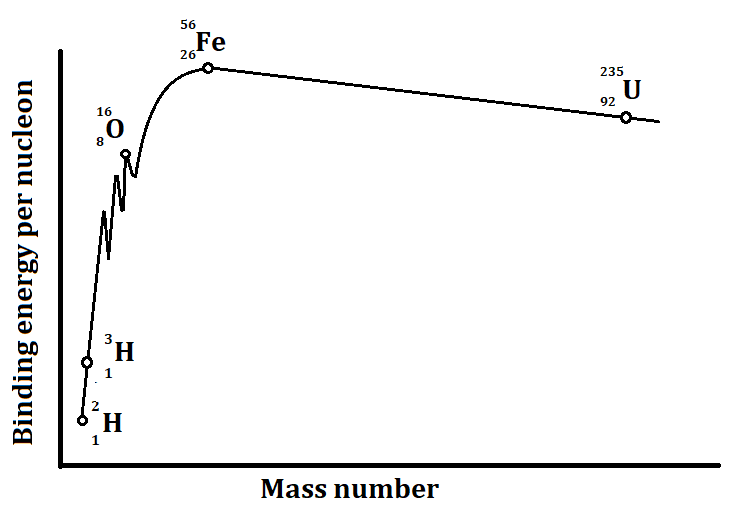
This graph tells us that:
1) ${}_{26}^{56}Fe$, which has an atomic mass of 56 has the maximum binding energy per nucleon. The energy is about 8.75 MeV.
2) ${}_1^2H$, has the minimum binding energy per nucleon.
3) The elements having atomic numbers between 30 and 170 (30 < A < 170) have nearly constant binding energy per nucleon.
4) We can also say that the binding energy per nucleon for both the light nuclei (A < 30) and heavy nuclei (A > 170) is low.
-Hence, we can now conclude from the above discussion and the graph that the highest binding energy per nucleon is of ${}_{26}^{56}Fe$.
So, the correct answer is “Option C”.
Note: The force of attraction between the nucleons is strong enough to produce a binding energy of few MeV per nucleon. But this nuclear force is short ranged which is why the binding energy per nucleon is constant for elements in the range of 30 < A < 170. Also this concept of binding energy is used in the nuclear fission reactions since the breakdown of an atom into two causes the nucleons to bond more tightly and there is release of energy.
Complete step by step answer:
-First of all let us see what binding energy is.
Binding energy is the energy released when some protons and neutrons are brought together to form a nucleus of some specific charge and mass. It is denoted as ${E_b}$. If we need to separate the protons and neutrons of a nucleus then we will need to provide this binding energy to the particles.
-Now let us talk about binding energy per nucleon.
Binding energy per nucleon is defined as the ratio of the binding energy of the nucleus to the number of nucleons present in the nucleus. It is mathematically written as:
${E_{bn}} = \dfrac{{{E_b}}}{A}$
Where, ${E_b}$ = binding energy; and A = number of nucleons in it.
-There is a graph which shows us the binding energy per nucleon for various nuclei. A simplified graph is shown below:

This graph tells us that:
1) ${}_{26}^{56}Fe$, which has an atomic mass of 56 has the maximum binding energy per nucleon. The energy is about 8.75 MeV.
2) ${}_1^2H$, has the minimum binding energy per nucleon.
3) The elements having atomic numbers between 30 and 170 (30 < A < 170) have nearly constant binding energy per nucleon.
4) We can also say that the binding energy per nucleon for both the light nuclei (A < 30) and heavy nuclei (A > 170) is low.
-Hence, we can now conclude from the above discussion and the graph that the highest binding energy per nucleon is of ${}_{26}^{56}Fe$.
So, the correct answer is “Option C”.
Note: The force of attraction between the nucleons is strong enough to produce a binding energy of few MeV per nucleon. But this nuclear force is short ranged which is why the binding energy per nucleon is constant for elements in the range of 30 < A < 170. Also this concept of binding energy is used in the nuclear fission reactions since the breakdown of an atom into two causes the nucleons to bond more tightly and there is release of energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




