
Among the given cations, ___ is most stable. (sec-butyl carbonium ion, tert-butyl carbonium ion, n-butyl carbonium ion and iso-butyl carbonium ion)
A. t-butyl carbonium ion
B. sec-butyl carbonium ion
C. n-butyl carbonium ion
D. iso-butyl carbonium ion
Answer
566.7k+ views
Hint: It is evident that the alkyl groups attached to a carbonium ion stabilizes it by the inductive effect and the process of hyperconjugation. The more is the number of alkyl groups attached to the central atom the more will be stability.
Step by step answer: In organic chemistry, we have seen different reactive species including free radicals, carbanions, carbocations which are also called carbonium ions and many others. Here, we will talk about carbonium ions which are basically organic cations in which there is an electron deficient, positively charged carbon atom. As the electron density is decreased here so we can say that the atoms or groups that can increase the same would stabilize the carbonium ions.
We can understand this in terms of inductive effect and hyperconjugation. In inductive effect, polarization of a sigma bond induces polarity in the adjacent sigma bonds. Based on the polarity, we can have a positive inductive effect when electron density is increased or negative in case it is decreased. It has been established that alkyl groups show positive inductive effect and are thus electron donating groups.
Now in terms of hyperconjugation, we can understand this as a stabilization effect by partial conjugation of sigma electrons of a bond with a system that is unsaturated such as positively charged carbon centre in a carbonium ion. So, we can say that higher numbers of alkyl groups would lead to more stabilization.
Now, let’s look at the given carbonium ions whose structure can be shown as follows:
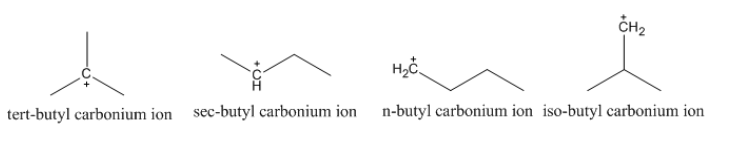
As we can see that in the given cations, it is tert-butyl carbonium ion that has maximum alkyl groups attached to the electron deficient carbon and thus is most stable.
Hence, the correct option is A.
Note: We can see that the structures of tert-butyl and iso-butyl might look similar at first glance but we have to pay attention to the carbon centre which is positively charged and is attached to three alkyl groups in first but two hydrogen atoms and one alkyl groups in the later cation.
Step by step answer: In organic chemistry, we have seen different reactive species including free radicals, carbanions, carbocations which are also called carbonium ions and many others. Here, we will talk about carbonium ions which are basically organic cations in which there is an electron deficient, positively charged carbon atom. As the electron density is decreased here so we can say that the atoms or groups that can increase the same would stabilize the carbonium ions.
We can understand this in terms of inductive effect and hyperconjugation. In inductive effect, polarization of a sigma bond induces polarity in the adjacent sigma bonds. Based on the polarity, we can have a positive inductive effect when electron density is increased or negative in case it is decreased. It has been established that alkyl groups show positive inductive effect and are thus electron donating groups.
Now in terms of hyperconjugation, we can understand this as a stabilization effect by partial conjugation of sigma electrons of a bond with a system that is unsaturated such as positively charged carbon centre in a carbonium ion. So, we can say that higher numbers of alkyl groups would lead to more stabilization.
Now, let’s look at the given carbonium ions whose structure can be shown as follows:

As we can see that in the given cations, it is tert-butyl carbonium ion that has maximum alkyl groups attached to the electron deficient carbon and thus is most stable.
Hence, the correct option is A.
Note: We can see that the structures of tert-butyl and iso-butyl might look similar at first glance but we have to pay attention to the carbon centre which is positively charged and is attached to three alkyl groups in first but two hydrogen atoms and one alkyl groups in the later cation.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




