
Among the species, which has the weakest carbon-oxygen bond:
A. $C{O_2}$
B. $C{H_3}COO$
C. CO
D. $CO_3^{2 - }$
Answer
573.3k+ views
Hint: The bond order of the compound depends on the bond strength of the compound. When bond order is less than the bond strength is weak and minimum bond dissociation energy is required to break the bond.
Complete step by step answer:
Bond order is defined as the number of bonding pairs of electrons forming the chemical bond present between the two atoms which determine the stability of the bond.
In $C{O_2}$, the carbon atom is bonded to two oxygen atoms by double bond. The structure is linear.
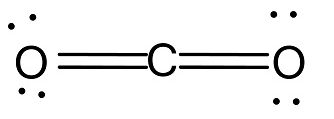
Total number of bonds is 4.
The number of bond groups between individual atoms is 2.
Then, the bond order is $\dfrac{4}{2} = 2$
In $C{H_3}COO$, carbon is bonded with methyl by a single bond, bonded with one oxygen atom by single bond and to other oxygen atoms by a double bond.
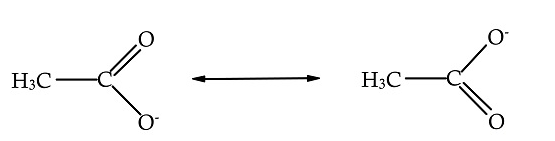
The total number of bonds between the atoms are 3.
The number of bond groups between individual atoms is 2.
The calculated bond order is $\dfrac{3}{2} = 1.5$
In CO, the carbon is bonded with oxygen by triple bond.
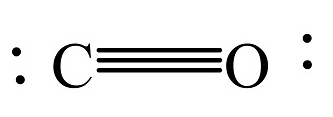
The bond order in CO is 3.
In $CO_3^{2 - }$, the carbon atom is bonded with two oxygen atoms by a single bond and other oxygen atom by double bond.
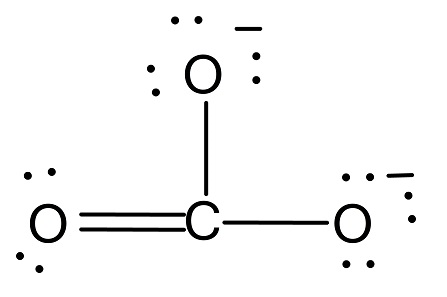
The total number of bonds is 4.
The number of bond groups between individual atoms is 3.
The calculated bond order is $\dfrac{4}{3} = 1.33$
The bond order is directly proportional to the bond strength. More is the bond order stronger is the bond.
As, the bond order of $CO_3^{2 - }$is 1.33 therefore the bond between carbon and oxygen is the weakest.
So, the correct answer is Option D.
Note: The bond order and bond length indicates the strength of the covalent bond. The bond order and bond length are inversely proportional to each other.
Complete step by step answer:
Bond order is defined as the number of bonding pairs of electrons forming the chemical bond present between the two atoms which determine the stability of the bond.
In $C{O_2}$, the carbon atom is bonded to two oxygen atoms by double bond. The structure is linear.

Total number of bonds is 4.
The number of bond groups between individual atoms is 2.
Then, the bond order is $\dfrac{4}{2} = 2$
In $C{H_3}COO$, carbon is bonded with methyl by a single bond, bonded with one oxygen atom by single bond and to other oxygen atoms by a double bond.

The total number of bonds between the atoms are 3.
The number of bond groups between individual atoms is 2.
The calculated bond order is $\dfrac{3}{2} = 1.5$
In CO, the carbon is bonded with oxygen by triple bond.
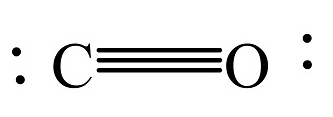
The bond order in CO is 3.
In $CO_3^{2 - }$, the carbon atom is bonded with two oxygen atoms by a single bond and other oxygen atom by double bond.

The total number of bonds is 4.
The number of bond groups between individual atoms is 3.
The calculated bond order is $\dfrac{4}{3} = 1.33$
The bond order is directly proportional to the bond strength. More is the bond order stronger is the bond.
As, the bond order of $CO_3^{2 - }$is 1.33 therefore the bond between carbon and oxygen is the weakest.
So, the correct answer is Option D.
Note: The bond order and bond length indicates the strength of the covalent bond. The bond order and bond length are inversely proportional to each other.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




