
Amphibole silicate structure has an 'x' number of corners shared per tetrahedron. The value of 'x' is
A.$2$
B.$2\dfrac{1}{2}$
C.3
D.4
Answer
585.3k+ views
Hint: Amphiboles refer to common rock-forming silicates which have hydroxyl groups in their structure and are considered to be hydrous silicates that are stable only in hydrous environments where water can be incorporated into the structure as $O{H^ - }$.
Complete step by step answer:
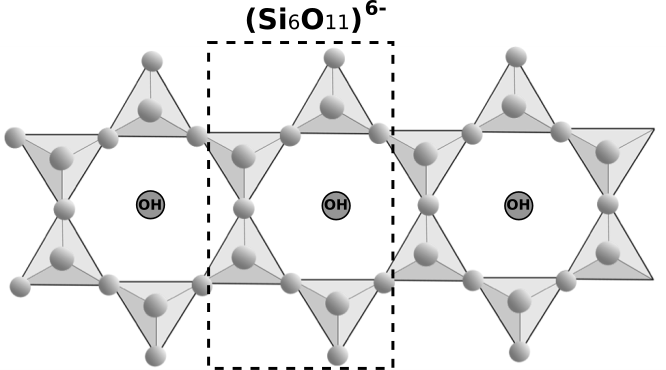
It has already been established that amphibole silicate structure has an 'x' number of corners shared per tetrahedron. The value of 'x' is $2\dfrac{1}{2}$. The structure of amphibole silicates is double-stranded cross-linked chains of composition \[{(S{i_4}{O_{11}})_n}^{6 - }\]. Two simple chains are joined together through the third oxygen atom of silicon tetrahedra \[{(Si{O_4})^{4 - }}\]. In each of the simple chains, two oxygen atoms per tetrahedra are shared. Examples of this structure include tremolite. In amphibole structure, structure of a basic unit \[{(S{i_6}{O_{11}})_n}^{6 - }\] is shown below:
Additional information: Other important types of silicates found in nature include:
Orthosilicates (or Nesosilicates): The ortho silicate ion is the strong conjugate base of weak orthosilicic acid as well as it will not persist in aqueous solutions. In nature, ortho silicates are rare and only found with cations which form highly insoluble salts.
-Pyro silicate (or Sorosilicates)
-Cyclic silicates (or Ring silicates)
-Chain silicates (or pyroxenes)
-Double chain silicate (or amphiboles)
-Sheet or phyllosilicates
-Three dimensional (or tecto) silicates
Note:
The chemical composition of amphibole silicates can be shown by the general formula:
\[{{\text{A}}_{{\text{0 - 1}}}}{{\text{B}}_{\text{2}}}{{\text{C}}_{\text{5}}}{{\text{T}}_{\text{8}}}{{\text{O}}_{{\text{22}}}}{\left( {{\text{OH, F, Cl}}} \right)_{\text{2}}}\], where
\[{\text{A}}\; = {\text{ }}Na,K\] ,
\[\;{\text{B}} = {\text{ }}Na,Zn,Li,Ca,Mn,F{e^{2 + }},Mg\] ,
\[{\text{C}} = {\text{ }}Mg,{\text{ }}F{e^{2 + }},{\text{ }}Mn,{\text{ }}Al,{\text{ }}F{e^{3 + }},Ti,Zn,{\text{ }}Cr\,{\text{ }}\;\],
\[{\text{T}}\; = {\text{ }}Si,{\text{ }}Al,{\text{ }}Ti\]
For most amphibole silicates the combination of the prismatic form and two diamond-shaped directions of cleavage at about ${56^o}$ and ${124^o}$ is the diagnostic feature.
Complete step by step answer:
It has already been established that amphibole silicate structure has an 'x' number of corners shared per tetrahedron. The value of 'x' is $2\dfrac{1}{2}$. The structure of amphibole silicates is double-stranded cross-linked chains of composition \[{(S{i_4}{O_{11}})_n}^{6 - }\]. Two simple chains are joined together through the third oxygen atom of silicon tetrahedra \[{(Si{O_4})^{4 - }}\]. In each of the simple chains, two oxygen atoms per tetrahedra are shared. Examples of this structure include tremolite. In amphibole structure, structure of a basic unit \[{(S{i_6}{O_{11}})_n}^{6 - }\] is shown below:

Additional information: Other important types of silicates found in nature include:
Orthosilicates (or Nesosilicates): The ortho silicate ion is the strong conjugate base of weak orthosilicic acid as well as it will not persist in aqueous solutions. In nature, ortho silicates are rare and only found with cations which form highly insoluble salts.
-Pyro silicate (or Sorosilicates)
-Cyclic silicates (or Ring silicates)
-Chain silicates (or pyroxenes)
-Double chain silicate (or amphiboles)
-Sheet or phyllosilicates
-Three dimensional (or tecto) silicates
Note:
The chemical composition of amphibole silicates can be shown by the general formula:
\[{{\text{A}}_{{\text{0 - 1}}}}{{\text{B}}_{\text{2}}}{{\text{C}}_{\text{5}}}{{\text{T}}_{\text{8}}}{{\text{O}}_{{\text{22}}}}{\left( {{\text{OH, F, Cl}}} \right)_{\text{2}}}\], where
\[{\text{A}}\; = {\text{ }}Na,K\] ,
\[\;{\text{B}} = {\text{ }}Na,Zn,Li,Ca,Mn,F{e^{2 + }},Mg\] ,
\[{\text{C}} = {\text{ }}Mg,{\text{ }}F{e^{2 + }},{\text{ }}Mn,{\text{ }}Al,{\text{ }}F{e^{3 + }},Ti,Zn,{\text{ }}Cr\,{\text{ }}\;\],
\[{\text{T}}\; = {\text{ }}Si,{\text{ }}Al,{\text{ }}Ti\]
For most amphibole silicates the combination of the prismatic form and two diamond-shaped directions of cleavage at about ${56^o}$ and ${124^o}$ is the diagnostic feature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




