
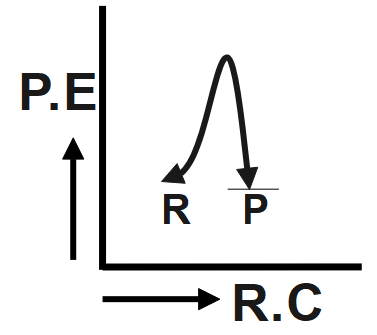
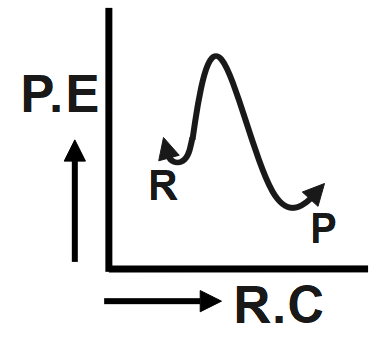
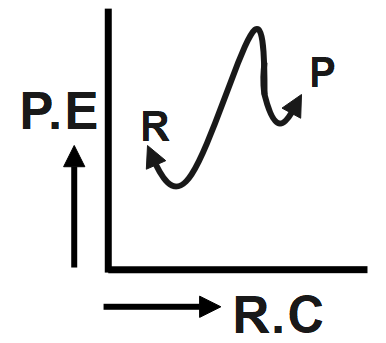
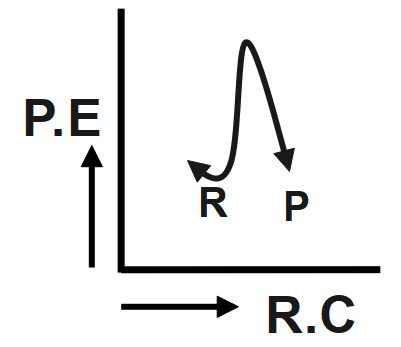
An endothermic reaction with high activation energy for the forward reaction is given by which diagram?
(A)
(B)
(C)
(D)




Answer
534.9k+ views
Hint: Activation energy of a chemical reaction is the amount of energy required to start a chemical reaction, activation energy is less for a spontaneous reaction and is high for a non-spontaneous reaction. Lesser the activation energy faster will be the rate of a reaction. A catalyst can be used to reduce the activation energy.
Complete step by step solution:
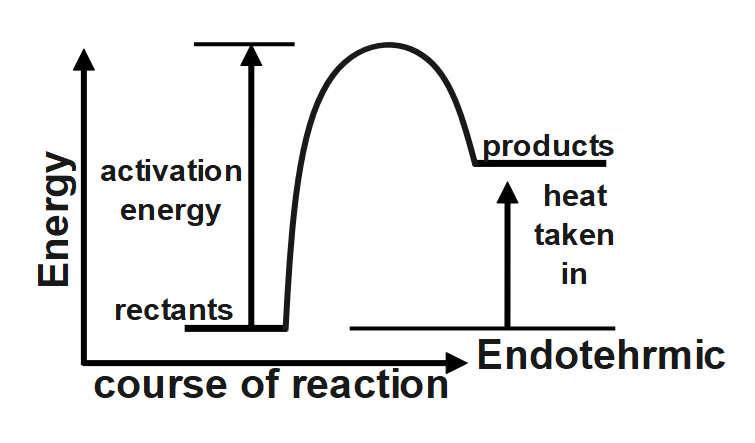
The reaction given in the question has the forward and backward reaction energy as and respectively. Now, since the reaction is an endothermic one, it would consume energy during its course of action, this implies that for the endothermic reaction the forward energy ( ${{E}_{b}}$ ) will be higher. And since we need to give energy for the reaction to occur this could mean that the reactant undergoing reaction is more stable than the product formed, hence we can conclude that the backward reaction energy ( ${{E}_{b}}$) will be less Therefore, we can say In an endothermic reaction, the overall energy is absorbed or taken in by the system and therefore, the energy of the product is higher than that of the reactant. An endothermic reaction with high activation energy for the forward reaction is given by the diagram C.
Note:
The clear knowledge of exothermic and endothermic reactions is the key to solve these types of questions. Catalyst can only catalyze a spontaneous reaction; it cannot catalyze a non-spontaneous reaction. it does not alter the Gibbs free energy of a reaction.
Complete step by step solution:
The reaction given in the question has the forward and backward reaction energy as and respectively. Now, since the reaction is an endothermic one, it would consume energy during its course of action, this implies that for the endothermic reaction the forward energy ( ${{E}_{b}}$ ) will be higher. And since we need to give energy for the reaction to occur this could mean that the reactant undergoing reaction is more stable than the product formed, hence we can conclude that the backward reaction energy ( ${{E}_{b}}$) will be less Therefore, we can say In an endothermic reaction, the overall energy is absorbed or taken in by the system and therefore, the energy of the product is higher than that of the reactant. An endothermic reaction with high activation energy for the forward reaction is given by the diagram C.

Note:
The clear knowledge of exothermic and endothermic reactions is the key to solve these types of questions. Catalyst can only catalyze a spontaneous reaction; it cannot catalyze a non-spontaneous reaction. it does not alter the Gibbs free energy of a reaction.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




