
An optically active hydrocarbon $X$ has molecular formula ${C_6}{H_{12}}$ , $X$ on catalytic hydrogenation gives optically inactive ${C_6}{H_{14}}$ , $X$ could be:
A.$3 - methyl - 1 - pentene$
B.$3 - methyl - 2 - pentene$
C.$4 - methyl - 2 - pentene$
D.$4 - ethyl - 1 - butene$
Answer
573.6k+ views
Hint:In this first we will find out whether the given molecular formula of hydrocarbon is an alkane, alkene or alkyne.Next we will see the catalytic hydrogenation of optically active hydrocarbon. Then accordingly we will see what is $X$ .
Complete step by step answer:
The molecular formula of the optically active hydrocarbon: ${C_6}{H_{12}}$
This molecular formula is the same as that of alkenes, that is: ${C_n}{H_{2n}}$.
So, from this we can say that the above formula of the hydrocarbon is of an alkene.
We got to know that this is an alkene, so we have to assume some of the possible structures for it that is optically active.
Before this we will see, what do you mean by optically active and optically inactive:
Optically active means that they are non super imposable mirror images of each other, are asymmetric and can rotate plane polarized light means it has chiral carbon atoms.
Optically inactive compound means that it does not rotate along the plane polarized light, does not have any chiral carbon atoms.
Possible Optically active structure of hydrocarbon with molecular formula ${C_6}{H_{12}}$ is:
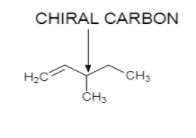
On catalytic hydrogenation, in the presence of ${H_2}/Pd$ it gives optically inactive compound with
molecular formula ${C_6}{H_{12}}$ :
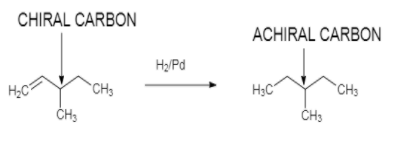
So from this we can assume that an optical active hydrocarbon $X$ with molecular formula ${C_6}{H_{12}}$ is $3 - methyl - 1 - pentene$.
So, the correct answer is option A: $3 - methyl - 1 - pentene$.
Note:
The compound that is optically active, that can rotate along the plane polarized light are called enantiomers. Enantiomers are of two types: racemic mixture(enantiomers in equal ratio),R,S configurations.
Complete step by step answer:
The molecular formula of the optically active hydrocarbon: ${C_6}{H_{12}}$
This molecular formula is the same as that of alkenes, that is: ${C_n}{H_{2n}}$.
So, from this we can say that the above formula of the hydrocarbon is of an alkene.
We got to know that this is an alkene, so we have to assume some of the possible structures for it that is optically active.
Before this we will see, what do you mean by optically active and optically inactive:
Optically active means that they are non super imposable mirror images of each other, are asymmetric and can rotate plane polarized light means it has chiral carbon atoms.
Optically inactive compound means that it does not rotate along the plane polarized light, does not have any chiral carbon atoms.
Possible Optically active structure of hydrocarbon with molecular formula ${C_6}{H_{12}}$ is:

On catalytic hydrogenation, in the presence of ${H_2}/Pd$ it gives optically inactive compound with
molecular formula ${C_6}{H_{12}}$ :

So from this we can assume that an optical active hydrocarbon $X$ with molecular formula ${C_6}{H_{12}}$ is $3 - methyl - 1 - pentene$.
So, the correct answer is option A: $3 - methyl - 1 - pentene$.
Note:
The compound that is optically active, that can rotate along the plane polarized light are called enantiomers. Enantiomers are of two types: racemic mixture(enantiomers in equal ratio),R,S configurations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




