
An organic compound (A) of molecular formula ${{\text{C}}_{\text{7}}}{{\text{H}}_{\text{8}}}$ on treatment with ${\text{C}}{{\text{l}}_{\text{2}}}$ in the presence of the sunlight gives compound (B) of molecular formula ${{\text{C}}_{\text{7}}}{{\text{H}}_{\text{7}}}{\text{Cl}}$. Compound (B) reacts with the Zn-Cu couple to give back the compound (A). Compound (B) on mild oxidation with ${\text{Cu(N}}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}$ gives compound (C) of molecular formula ${{\text{C}}_{\text{7}}}{{\text{H}}_{\text{6}}}{\text{O}}$. Identify the compound A, B, C. Explain the reaction.
Answer
544.5k+ views
Hint:In the above question, we have to find the organic compound A, B and C. Since the molecular formula ${{\text{C}}_{\text{7}}}{{\text{H}}_{\text{8}}}$ which is a representation of toluene is given at the beginning, we can start solving the reaction occurred from the beginning. The first reaction is chlorination, followed by de-chlorination. The third reaction is oxidation.
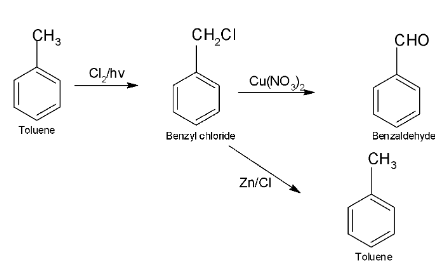
Complete step-by-step answer:In the above question, the molecular formula of A is given as ${{\text{C}}_{\text{7}}}{{\text{H}}_{\text{8}}}$ which is representation of ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{ - C}}{{\text{H}}_{\text{3}}}$ (which is the formula of toluene).
So, compound A is toluene.
Toluene when reacts with ${\text{C}}{{\text{l}}_{\text{2}}}$ in the presence of sunlight removes the H atom from toluene and chlorine gets attached to it which is also known as photo chlorination.
So, compound B is benzyl chloride.
When benzyl chloride reacts with Zn-Cu, de-chlorination takes place which leads to formation of toluene which is compound a.
When benzyl chloride reacts with mild oxidant, oxygen atom replaces chlorine from benzyl chloride and leads to formation of benzaldehyde.
The whole reaction can be illustrated as:
Note:In these types of questions, we have to look where the compound molecular formula is given. If it is given in the beginning, we can start solving it as usual. If the molecular formula or molecule is given at the end, we must start solving the equation backward.
Complete step-by-step answer:In the above question, the molecular formula of A is given as ${{\text{C}}_{\text{7}}}{{\text{H}}_{\text{8}}}$ which is representation of ${{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}{\text{ - C}}{{\text{H}}_{\text{3}}}$ (which is the formula of toluene).
So, compound A is toluene.
Toluene when reacts with ${\text{C}}{{\text{l}}_{\text{2}}}$ in the presence of sunlight removes the H atom from toluene and chlorine gets attached to it which is also known as photo chlorination.
So, compound B is benzyl chloride.
When benzyl chloride reacts with Zn-Cu, de-chlorination takes place which leads to formation of toluene which is compound a.
When benzyl chloride reacts with mild oxidant, oxygen atom replaces chlorine from benzyl chloride and leads to formation of benzaldehyde.
The whole reaction can be illustrated as:

Note:In these types of questions, we have to look where the compound molecular formula is given. If it is given in the beginning, we can start solving it as usual. If the molecular formula or molecule is given at the end, we must start solving the equation backward.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




