
An organic compound \[A\left( {{C_4}{H_9}Cl} \right)\] on reaction with \[Na\]\[/\]diethyl ether gives a hydrocarbon which on monochlorination gives only one chloro derivative then, \[A\] is:
\[A.\,\,isobutyl\,chloride\]
\[B.\,\,secondary\,butyl\,chloride\]
\[C.\,\,tertiary\,butyl\,chloride\]
\[D.\,\,n - butyl\,chloride\]
Answer
579k+ views
Hint:We should know the concept of Wurtz’s reaction. It involves the reaction of alkyl halides with Na in ethanol to form higher alkanes. This compound is sparingly soluble in water, miscible with alcohol and water. \[tetramethylbutane\]
Complete step by step answer:
The organic chemical reaction where Sodium metal reacts with two alkyl halides in the presence of dry ether to form alkane is called Wurtz’s reaction. The term hydrocarbon indicates symmetrical hydrocarbon Alkane.
The general equation of Wurtz’s reaction is shown below,
\[R - X\, + \,2Na\, + R - X\,\xrightarrow{{dry\,ether}}\,R - R\, + \,2NaX\]
Let us see another example of Wurtz’s reaction,
The reaction between methyl bromide and sodium in presence of anhydrous ether gives Ethane and byproduct.
\[2C{H_3}Br\, + \,2Na \to \,{C_2}{H_6}\, + \,2NaBr\]
The given organic compound \[A\left( {{C_4}{H_9}Cl} \right)\] on reaction with Sodium or diethyl ether gives a hydrocarbon which is \[2,2,3,3 - \] tetramethylbutane. Its structure is given below,
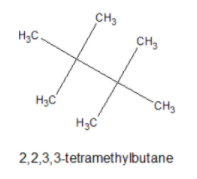
Chlorine replaces the one type of \[H\] atom in this compound and gives only a monochloro derivative. So the compound \[A\left( {{C_4}{H_9}Cl} \right)\] is tertiary butyl chloride.
Therefore the correct answer is option C.
ADDITIONAL INFORMATION:
The mechanism of Wurtz’s reaction involves a free radical species which is a part of metal \[ - \] halogen exchange.\[{\left( {C{H_3}} \right)_3}CCl\] is the chemical formula of tertiary butyl chloride.
Dry ether is a very good poly aprotic solvent. Dry ether is completely free from water.
Note:
Methane cannot be prepared via Wurtz’s reaction since the product of this reaction must have at least two carbon atoms. Tertiary alkyl halides do not respond to this reaction. Only symmetric alkane can be synthesized through this method. We should note that sodium metal used in Wurtz’s reaction is highly reactive, so we need a solvent which does not react with sodium metal.
Complete step by step answer:
The organic chemical reaction where Sodium metal reacts with two alkyl halides in the presence of dry ether to form alkane is called Wurtz’s reaction. The term hydrocarbon indicates symmetrical hydrocarbon Alkane.
The general equation of Wurtz’s reaction is shown below,
\[R - X\, + \,2Na\, + R - X\,\xrightarrow{{dry\,ether}}\,R - R\, + \,2NaX\]
Let us see another example of Wurtz’s reaction,
The reaction between methyl bromide and sodium in presence of anhydrous ether gives Ethane and byproduct.
\[2C{H_3}Br\, + \,2Na \to \,{C_2}{H_6}\, + \,2NaBr\]
The given organic compound \[A\left( {{C_4}{H_9}Cl} \right)\] on reaction with Sodium or diethyl ether gives a hydrocarbon which is \[2,2,3,3 - \] tetramethylbutane. Its structure is given below,

Chlorine replaces the one type of \[H\] atom in this compound and gives only a monochloro derivative. So the compound \[A\left( {{C_4}{H_9}Cl} \right)\] is tertiary butyl chloride.
Therefore the correct answer is option C.
ADDITIONAL INFORMATION:
The mechanism of Wurtz’s reaction involves a free radical species which is a part of metal \[ - \] halogen exchange.\[{\left( {C{H_3}} \right)_3}CCl\] is the chemical formula of tertiary butyl chloride.
Dry ether is a very good poly aprotic solvent. Dry ether is completely free from water.
Note:
Methane cannot be prepared via Wurtz’s reaction since the product of this reaction must have at least two carbon atoms. Tertiary alkyl halides do not respond to this reaction. Only symmetric alkane can be synthesized through this method. We should note that sodium metal used in Wurtz’s reaction is highly reactive, so we need a solvent which does not react with sodium metal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




