
What is the $ \angle O-O-O $ bond angle in ozone?
Answer
491.7k+ views
Hint: One of the most common instances of the Lewis structure is ozone. Ozone is made up of three oxygen atoms. In core chemistry equations, it is represented as $ {{O}_{3}} $ . It is critical to grasp the Lewis structure of the Ozone molecule in order to comprehend its hybridization, polarity, and molecular geometry. The octet rule governs Lewis structure. The octet rule says that for a molecule to be stable, it must have eight electrons in its outer shell or orbit. The number of valence electrons in a molecule can be determined using Lewis structure. The electrons that engage in bond formation and nonbonding electron pairs are known as valence electrons.
Complete answer:
Each molecule of Oxygen in Ozone or $ {{O}_{3}} $ has six valence electrons.
The total number of valence electrons Is $ \text{ 6 x 3=18} $ since there are three oxygen molecules. As a result, the Ozone molecule has a total of 18 valence electrons accessible.
Because Ozone has just one central Oxygen atom with eight electrons in its outermost shell, the central atom's hybridization will be $ s{{p}^{2}} $ . The 2s orbital has two electrons, but the 2p orbitals have six electrons in each of the three 2p orbitals. Because there are electrons in one s orbital and two p orbitals, the centre oxygen atom's hybridization becomes $ s{{p}^{2}} $ . Hybridization is also present in the other two oxygen atoms. Because there is one lone pair of electrons that produces resonance in the structure of Ozone, one will have $ s{{p}^{2}} $ hybridization and the other will have sp3 hybridization. Ozone possesses $ s{{p}^{2}} $ hybridization because we always regard the centre atom's hybridization to be the ultimate hybridization.
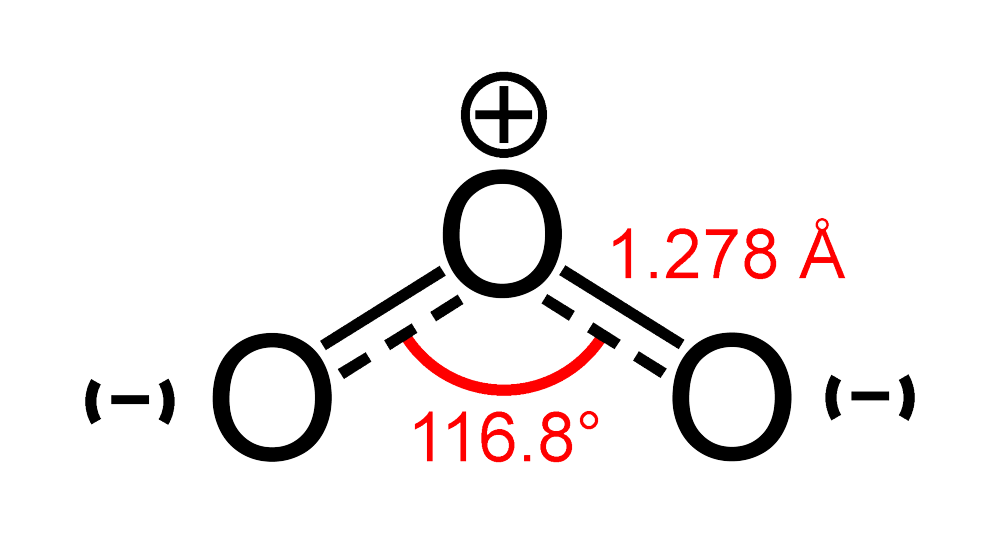
We can now calculate the molecular geometry of Ozone since the hybridization of the molecule affects its form. Because ozone is $ s{{p}^{2}} $ hybridised, it should have a trigonal planar form. The angle between the molecules is smaller than $ {{120}^{o}} $ degrees since the structure of Ozone contains resonance and one lone pair of electrons. Between bonding electrons, there is always a repulsive force that is smaller than the repulsion between a lone pair and bonding electrons. Because there are just one pair of lone electrons in this scenario, the angle decreases from $ {{120}^{o}} $ to $ {{116.8}^{o}} $ degrees.
Note:
Every molecule's polarity is determined by its molecular geometry. Because of its valence electrons, the Ozone molecule is twisted here. Because of their $ s{{p}^{2}} $ hybridization, all three Oxygen molecules are not linear. Because the molecules aren't in a straight line, their dipole interactions aren't neutralised, and the molecule has a net dipole. As a result, Ozone has polarity, and it may be argued that Ozone is polar. The polarity of Ozone is owing to a single pair of electrons on the core atom.
Complete answer:
Each molecule of Oxygen in Ozone or $ {{O}_{3}} $ has six valence electrons.
The total number of valence electrons Is $ \text{ 6 x 3=18} $ since there are three oxygen molecules. As a result, the Ozone molecule has a total of 18 valence electrons accessible.
Because Ozone has just one central Oxygen atom with eight electrons in its outermost shell, the central atom's hybridization will be $ s{{p}^{2}} $ . The 2s orbital has two electrons, but the 2p orbitals have six electrons in each of the three 2p orbitals. Because there are electrons in one s orbital and two p orbitals, the centre oxygen atom's hybridization becomes $ s{{p}^{2}} $ . Hybridization is also present in the other two oxygen atoms. Because there is one lone pair of electrons that produces resonance in the structure of Ozone, one will have $ s{{p}^{2}} $ hybridization and the other will have sp3 hybridization. Ozone possesses $ s{{p}^{2}} $ hybridization because we always regard the centre atom's hybridization to be the ultimate hybridization.
We can now calculate the molecular geometry of Ozone since the hybridization of the molecule affects its form. Because ozone is $ s{{p}^{2}} $ hybridised, it should have a trigonal planar form. The angle between the molecules is smaller than $ {{120}^{o}} $ degrees since the structure of Ozone contains resonance and one lone pair of electrons. Between bonding electrons, there is always a repulsive force that is smaller than the repulsion between a lone pair and bonding electrons. Because there are just one pair of lone electrons in this scenario, the angle decreases from $ {{120}^{o}} $ to $ {{116.8}^{o}} $ degrees.

Note:
Every molecule's polarity is determined by its molecular geometry. Because of its valence electrons, the Ozone molecule is twisted here. Because of their $ s{{p}^{2}} $ hybridization, all three Oxygen molecules are not linear. Because the molecules aren't in a straight line, their dipole interactions aren't neutralised, and the molecule has a net dipole. As a result, Ozone has polarity, and it may be argued that Ozone is polar. The polarity of Ozone is owing to a single pair of electrons on the core atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




