
When aniline is nitrated with nitrating mixture in ice cold condition, the major product obtained is:
a) p-nitroaniline
b) 2,4-dinitroaniline
c) o-nitroaniline
d) m-nitroaniline
Answer
597k+ views
Hint: Nitration of aromatic compounds is generally done using a nitrating mixture which consists of Conc. $ HN{ O }_{ 3 }$ and Conc. $ { H }_{ 2 }{ SO }_{ 4 }$ at low temperatures. $ -N{ H }_{ 2 }$ group present in aniline is a strong activating group and is ortho and para directing.
Complete step by step answer:
First let us understand the nature of aniline in terms of its chemical reaction.
i) Scheme-1-$ -N{ H }_{ 2 }$ group present in aniline is a strong activating group and is ortho and para directing due to its strong +R effect. Nitric acid is a strong oxidizing agent. As a result, when the nitration of aniline is carried out, it not only gives nitration products but also some oxidation products. However, under controlled conditions, if the nitration of aniline is carried out, then the major products are p-nitroaniline and m-nitroaniline. The reaction is given below:
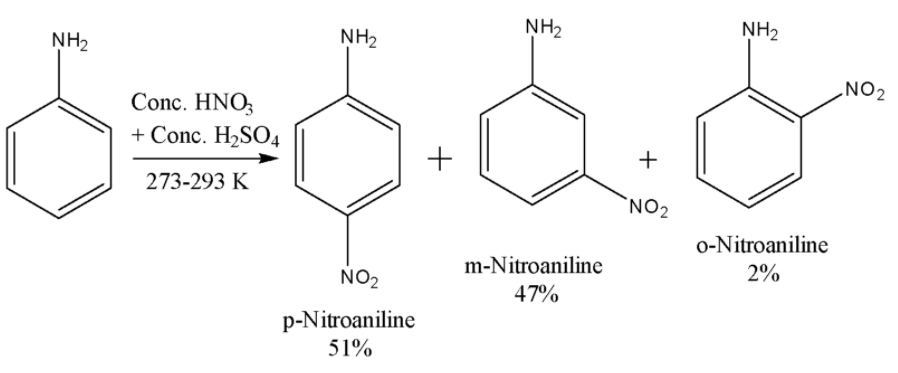
Here the major product is p-Nitroaniline. The m-Nitroaniline is also formed in a significant quantity because here very strong acidic conditions are being used due to which some of the aniline molecules get protonated to anilinium ion. The $-N{ H }_{ 3 }^{ + }$ group is electron withdrawing and m-directing due to which we get a significant amount of the meta product.
ii) Scheme-2-In order to get rid of the meta product, we protect the $ -N{ H }_{ 2 }$ group in aniline by acetylation due to which it no longer gets protonated but still remains ortho, para directing. After the nitration reaction, the acetyl group is removed by hydrolysis. The reaction is given below:
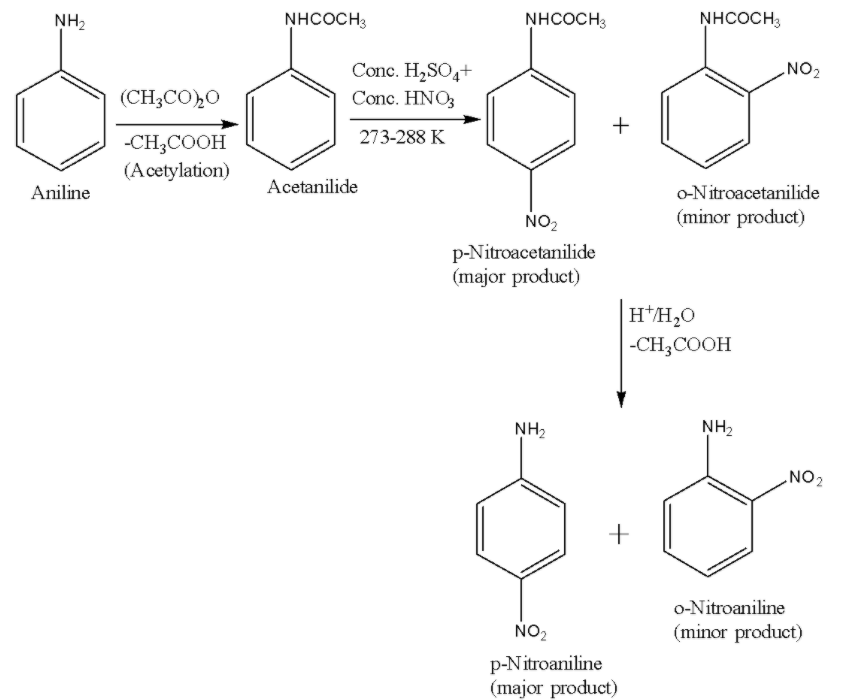
Therefore, in both of the reaction schemes 1 and 2, it is clear that the major product is p-Nitroaniline.
Hence the correct option is (a) p-nitroaniline.
Note: The o-Nitroaniline is always the minor product in both the reaction schemes 1 and 2. This is because of the steric hindrance at the ortho position offered by the group due to which the nucleophilic substitution becomes difficult at the ortho position.
Complete step by step answer:
First let us understand the nature of aniline in terms of its chemical reaction.
i) Scheme-1-$ -N{ H }_{ 2 }$ group present in aniline is a strong activating group and is ortho and para directing due to its strong +R effect. Nitric acid is a strong oxidizing agent. As a result, when the nitration of aniline is carried out, it not only gives nitration products but also some oxidation products. However, under controlled conditions, if the nitration of aniline is carried out, then the major products are p-nitroaniline and m-nitroaniline. The reaction is given below:

Here the major product is p-Nitroaniline. The m-Nitroaniline is also formed in a significant quantity because here very strong acidic conditions are being used due to which some of the aniline molecules get protonated to anilinium ion. The $-N{ H }_{ 3 }^{ + }$ group is electron withdrawing and m-directing due to which we get a significant amount of the meta product.
ii) Scheme-2-In order to get rid of the meta product, we protect the $ -N{ H }_{ 2 }$ group in aniline by acetylation due to which it no longer gets protonated but still remains ortho, para directing. After the nitration reaction, the acetyl group is removed by hydrolysis. The reaction is given below:

Therefore, in both of the reaction schemes 1 and 2, it is clear that the major product is p-Nitroaniline.
Hence the correct option is (a) p-nitroaniline.
Note: The o-Nitroaniline is always the minor product in both the reaction schemes 1 and 2. This is because of the steric hindrance at the ortho position offered by the group due to which the nucleophilic substitution becomes difficult at the ortho position.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




