
Aniline is treated with $B{{r}_{2}}$ water at room temperature to give the following product:
(a)
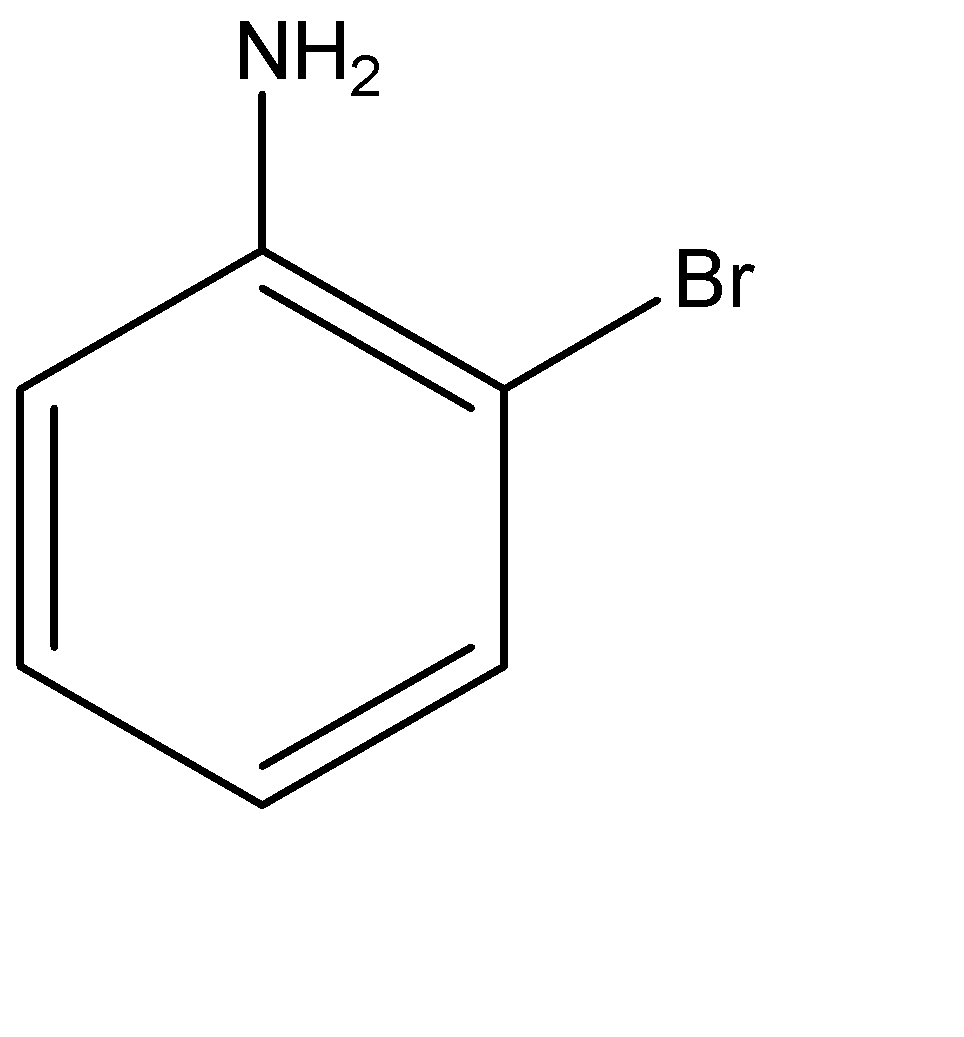
(b)
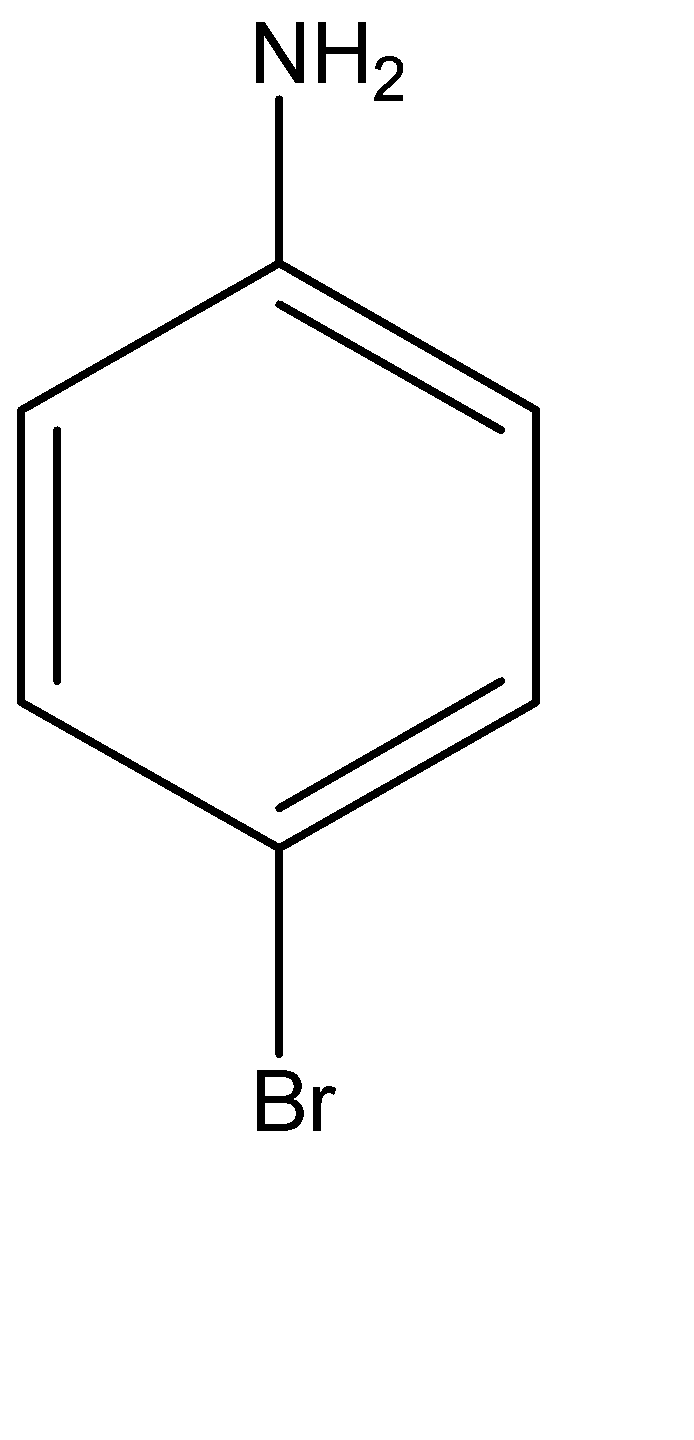
(c)
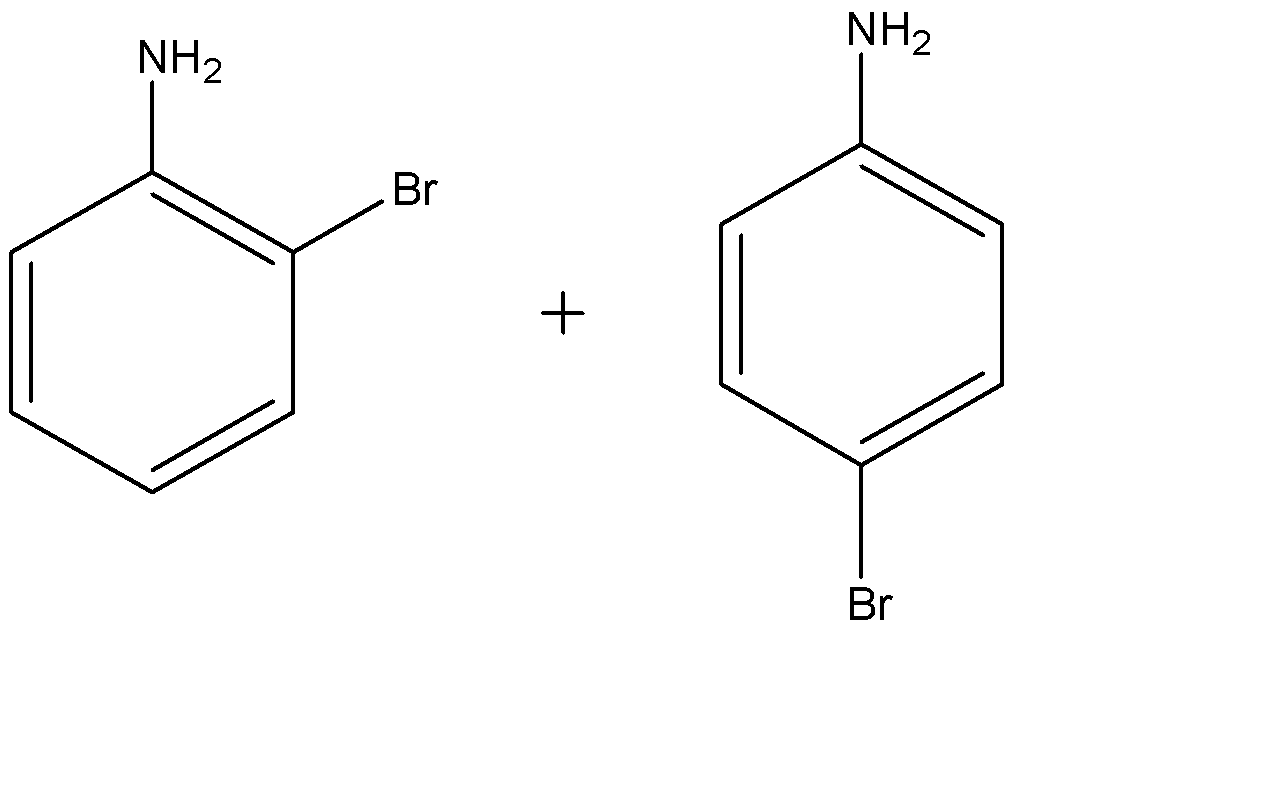
(d)
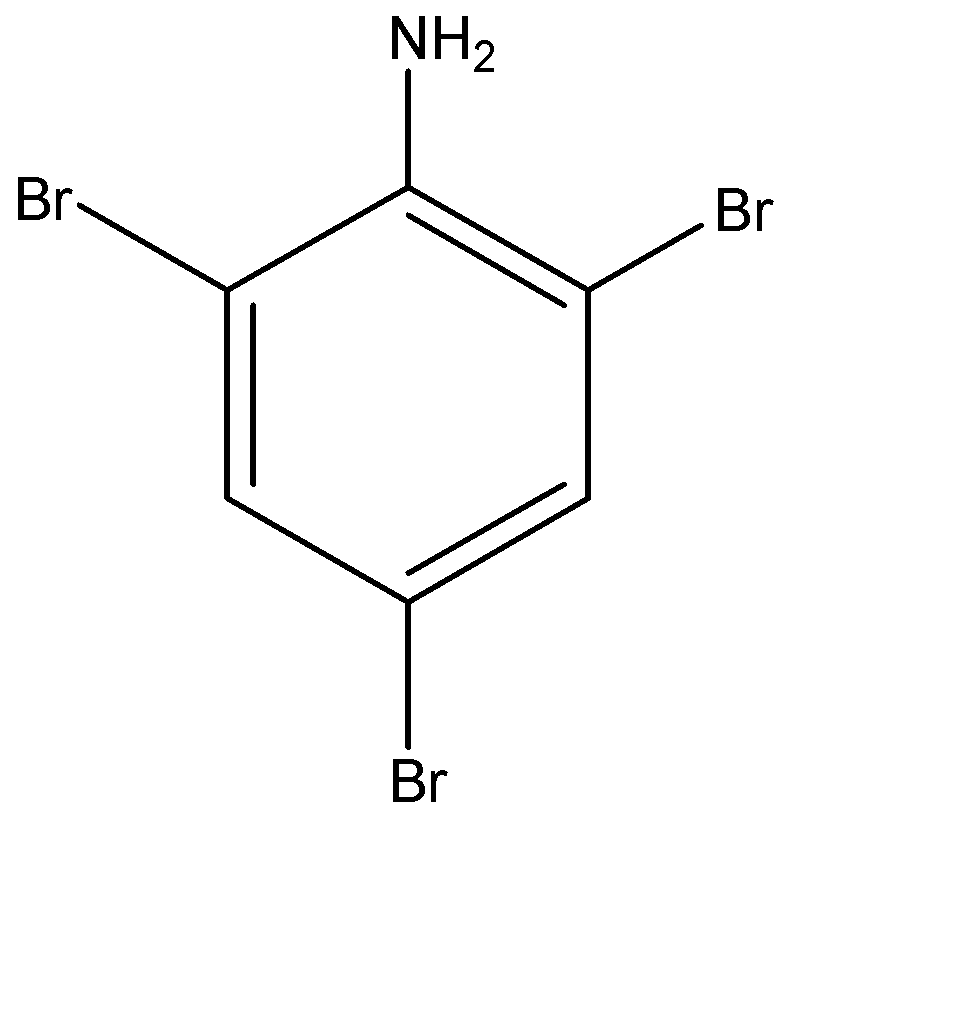




Answer
517.2k+ views
Hint: As we know that aniline is an organic compound that consists of a phenyl group attached to an amino group. It is the simplest aromatic amine available. It is industrially very significant and used as a starting reaction in organic synthesis of various compounds. So here we are to tell the compound formed when aniline is treated with Bromine water at room temperature.
Complete answer:
Let us discuss about reactive nature of aniline followed by the reaction as follows:-
Just like phenols, aniline does give electrophilic substitution reaction. Due to its high reactivity, it can also be categorized as an enamine. This is because aniline has a good electron donating ability to the electrophilic species. This can be seen in the reaction of aniline with bromine water at room temperature as follows:-
-Bromination
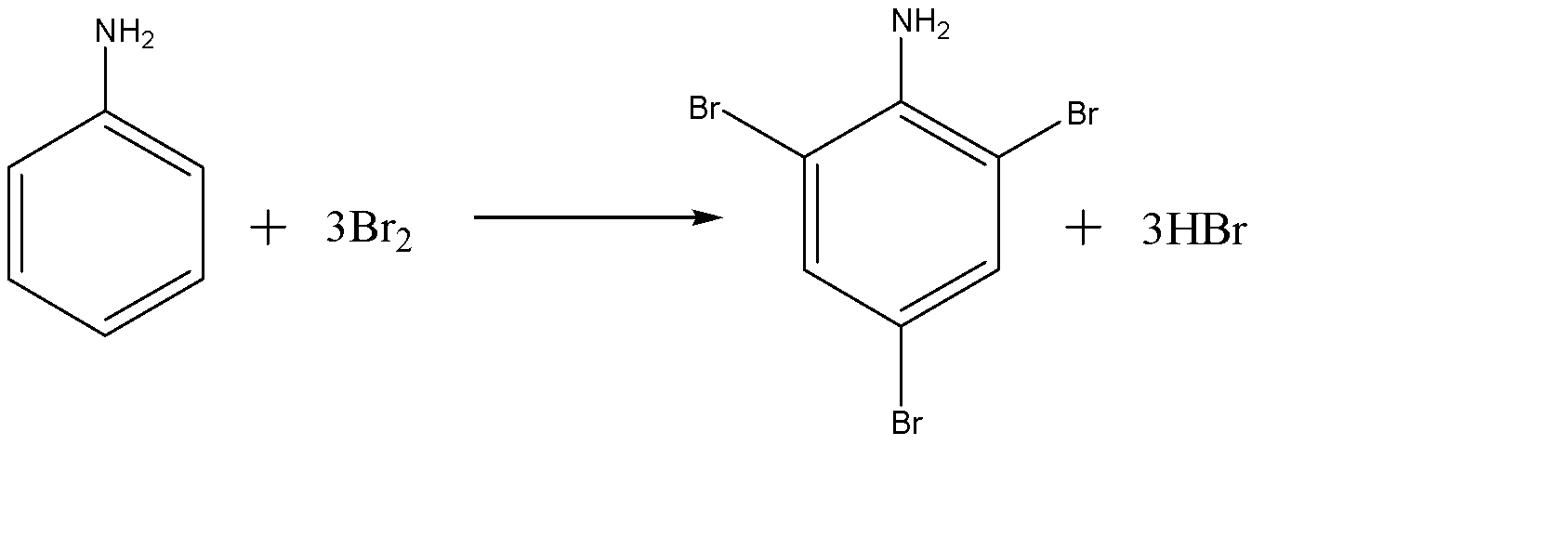
The benzene ring of aniline is so highly activated due to strong mesomeric effect amino group that when it is treated with bromine water ($B{{r}_{2}}$ ) at room temperature, electrophilic substitution takes place at all para and ortho positions which results in the formation of 2,4,6-tribromoaniline.
-The formation of 2,4,6-tribromoaniline can be seen because a white colour precipitate is formed in the solution and also the yellowish orange colour of bromine water also disappears at the same time.
-Therefore we can say that, aniline is treated with $B{{r}_{2}}$ water at room temperature to give following product:-
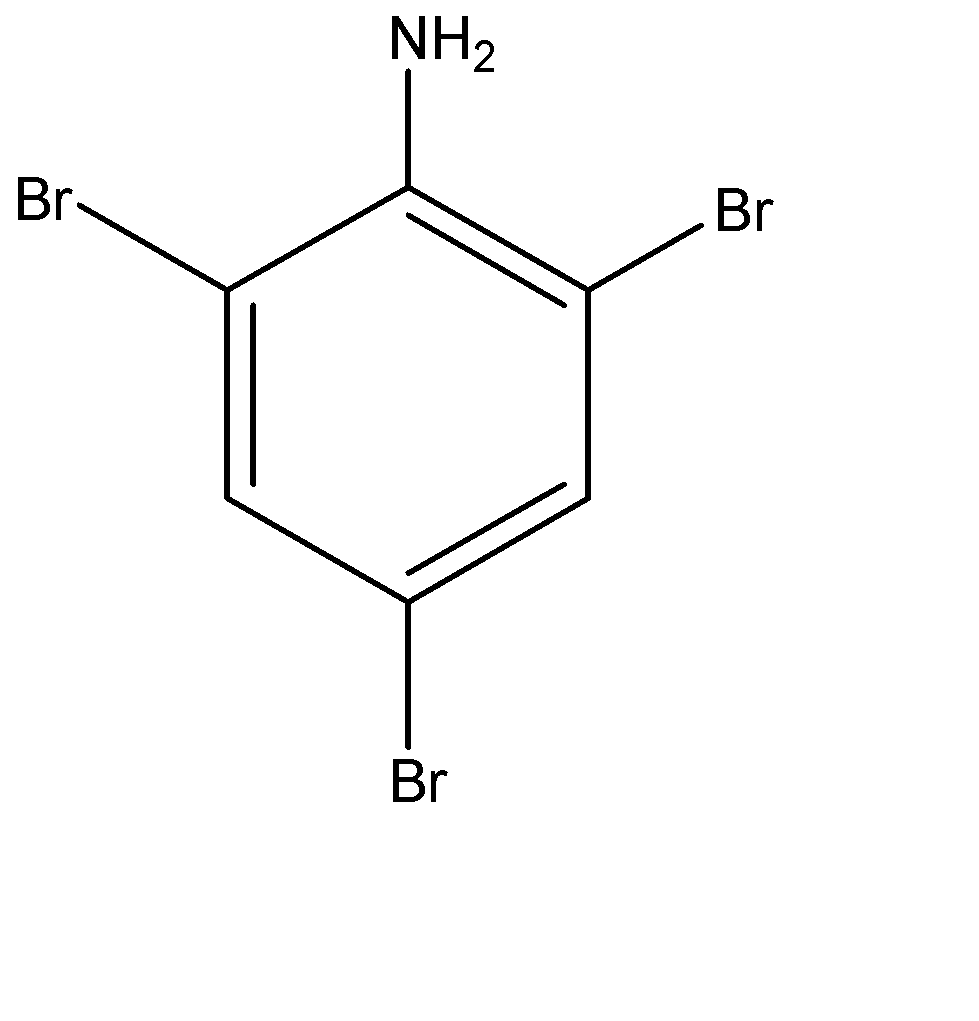
(d)
Note:
-To obtain a monosubstituted product, we need to decrease the effect of the amino group by acetylating this group before the substitution reaction.
-Also study and learn all types of reaction shown by aniline as it is an important reactant in most of the organic synthesis.
Complete answer:
Let us discuss about reactive nature of aniline followed by the reaction as follows:-
Just like phenols, aniline does give electrophilic substitution reaction. Due to its high reactivity, it can also be categorized as an enamine. This is because aniline has a good electron donating ability to the electrophilic species. This can be seen in the reaction of aniline with bromine water at room temperature as follows:-
-Bromination

The benzene ring of aniline is so highly activated due to strong mesomeric effect amino group that when it is treated with bromine water ($B{{r}_{2}}$ ) at room temperature, electrophilic substitution takes place at all para and ortho positions which results in the formation of 2,4,6-tribromoaniline.
-The formation of 2,4,6-tribromoaniline can be seen because a white colour precipitate is formed in the solution and also the yellowish orange colour of bromine water also disappears at the same time.
-Therefore we can say that, aniline is treated with $B{{r}_{2}}$ water at room temperature to give following product:-
(d)

Note:
-To obtain a monosubstituted product, we need to decrease the effect of the amino group by acetylating this group before the substitution reaction.
-Also study and learn all types of reaction shown by aniline as it is an important reactant in most of the organic synthesis.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




