
How anisole reacts with bromine in presence of ethanoic acid? Write the chemical equation for the reaction.
Answer
522.2k+ views
Hint: Anisole is an electron withdrawing group, it undergoes electrophilic substitution. The halogenation will take place only at the ortho and para positions for anisole and not at the meta position.
Complete Step by Step Solution: Anisole is an organic compound and we also know it by the name methoxybenzene. The chemical formula of anisole is ${{C}_{6}}{{H}_{5}}OC{{H}_{3}}$.
The methoxy group present in anisole will donate its electron density of the oxygen atom to the benzene ring and therefore the electron density on the benzene ring will increase and it will undergo electrophilic aromatic substitution, resulting in halogenation.
As we know, the methoxy group present in anisole makes it an ortho-para directing group, which means the attacking atom/group will be attached to the ortho or para positions with respect to the methoxy group in the ring.
Ethanoic acid, as we know it by the name acetic acid acts as a polar solvent for dissolving anisole.
We can write the reaction of bromination of anisole as-
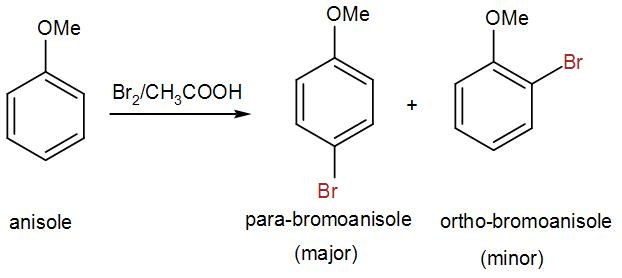
Here, we get two products, para-bromoanisole which we can also write as 1-bromo-2-methoxybenzene and the other product is ortho-bromoanisole or 1-bromo-4-methoxybenzene.
Among these two, as we can see para-bromoanisole is the major product and the yield is around 90% for it and the ortho-bromoanisole is the minor product with a very low yield. This is due to the steric hindrance in the ortho position as a bulky methoxy group is attached to the benzene ring.
Note: As we can see in the above discussion we mentioned that electron withdrawing groups increases the electron density at ortho and para position but the reason behind it can be understood from the resonance hybrid structures of anisole-
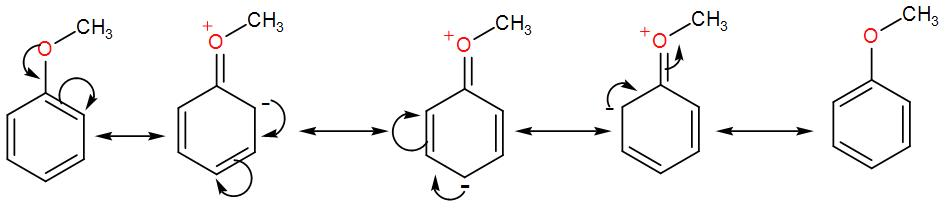
As we can see, the negative charge is passing through the ortho and the para positions therefore, these are the activated positions hence we get an ortho and a para product.
Complete Step by Step Solution: Anisole is an organic compound and we also know it by the name methoxybenzene. The chemical formula of anisole is ${{C}_{6}}{{H}_{5}}OC{{H}_{3}}$.
The methoxy group present in anisole will donate its electron density of the oxygen atom to the benzene ring and therefore the electron density on the benzene ring will increase and it will undergo electrophilic aromatic substitution, resulting in halogenation.
As we know, the methoxy group present in anisole makes it an ortho-para directing group, which means the attacking atom/group will be attached to the ortho or para positions with respect to the methoxy group in the ring.
Ethanoic acid, as we know it by the name acetic acid acts as a polar solvent for dissolving anisole.
We can write the reaction of bromination of anisole as-

Here, we get two products, para-bromoanisole which we can also write as 1-bromo-2-methoxybenzene and the other product is ortho-bromoanisole or 1-bromo-4-methoxybenzene.
Among these two, as we can see para-bromoanisole is the major product and the yield is around 90% for it and the ortho-bromoanisole is the minor product with a very low yield. This is due to the steric hindrance in the ortho position as a bulky methoxy group is attached to the benzene ring.
Note: As we can see in the above discussion we mentioned that electron withdrawing groups increases the electron density at ortho and para position but the reason behind it can be understood from the resonance hybrid structures of anisole-

As we can see, the negative charge is passing through the ortho and the para positions therefore, these are the activated positions hence we get an ortho and a para product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




