
What are allotropes? Name any two allotropic forms of carbon. Give one use of it.
Answer
528.3k+ views
Hint: The allotropes are the various structures of the same element. It also refers to one or more physical forms of a chemical element but may show the difference in physical and chemical properties. Carbon shows the catenation property. Therefore it has allotropes. The most common allotropes of carbon are graphite and diamond. The graphite is a structure of $\text{s}{{\text{p}}^{\text{2}}}$ hybridized carbon atoms, and a diamond is a network of $\text{s}{{\text{p}}^{3}}$ hybridized carbon atoms.
Complete step by step answer:
First of all, we can check the meaning of the word ‘Allotropy’. The word ‘Allotropy’ is a combination of two words ‘Allos’ meaning ‘other’ and ‘tropos’ meaning ‘form’. So, it has a word meaning ‘other form’.
As the meaning suggests, the term allotrope refers to one or more physical forms of a chemical element that occurs in the same physical state. Allotropes may show differences in chemical and physical properties. The carbon has a property to form a bond with another carbon atom. This is called catenation. Due to this carbon exhibits the allotropy.
Let us have a look at the most widely known allotropes of carbon:
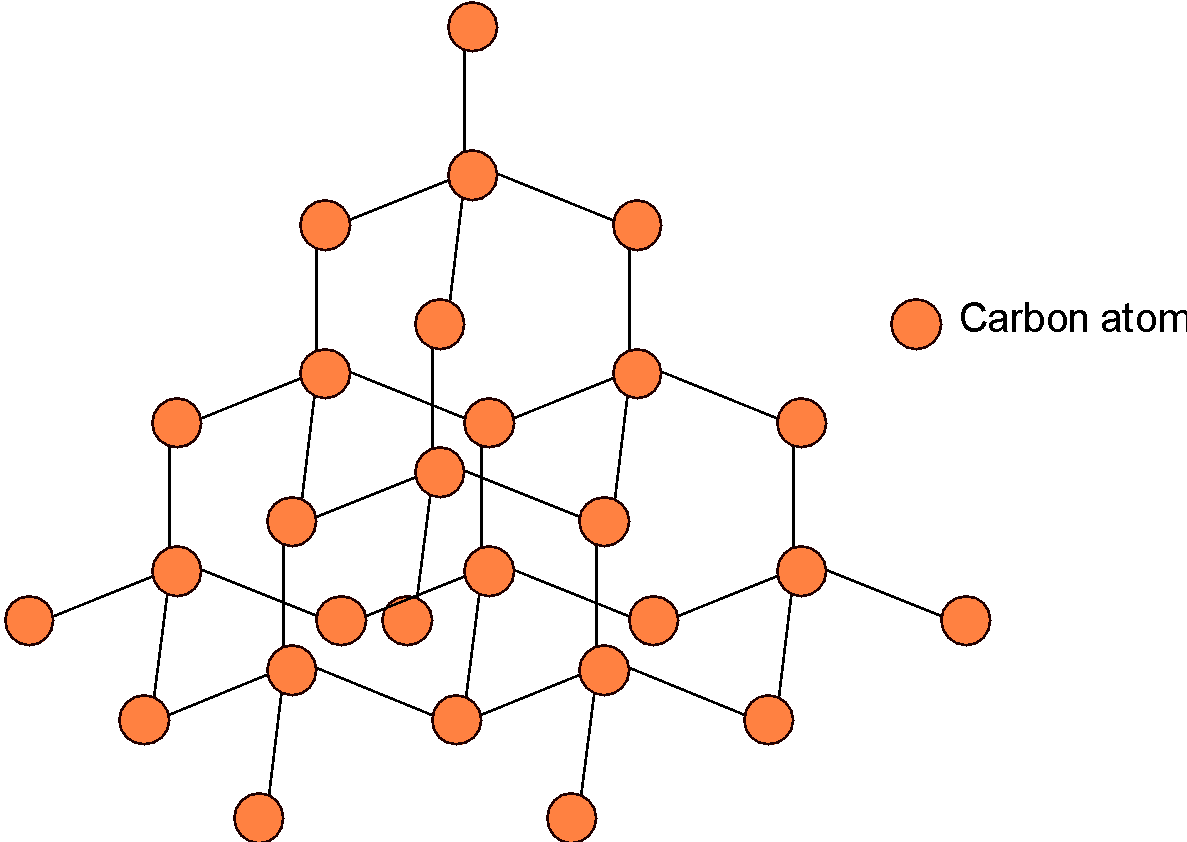
1) Graphite: -It is the purest form of carbon. It is composed of 2-D hexagonal layers of carbon atoms which are arranged in a hexagonal structure.
-Graphite is soft, slippery, and black solid. This property allows the layers of structure to slip over.
-Each carbon in graphite forms three bonds with the adjacent carbon atoms. Therefore, each carbon is $\text{s}{{\text{p}}^{\text{2}}}$ hybridized. The pi-electrons are localized over the structure. Therefore, graphite is a good conductor of electricity.
-Graphite exists in $\text{ }\!\!\alpha\!\!\text{ }$ and $\text{ }\!\!\beta\!\!\text{ }$ forms. In $\text{ }\!\!\alpha\!\!\text{ }$ form, the layers are in sequence of \[\text{ABAB}\] and in $\text{ }\!\!\beta\!\!\text{ }$ form the layers are arranged as\[\text{ABCABC}\] .
Use: It is used to make the lead of a pencil.it is also used as the lubricant.
-The structure of the graphite is as shown below,
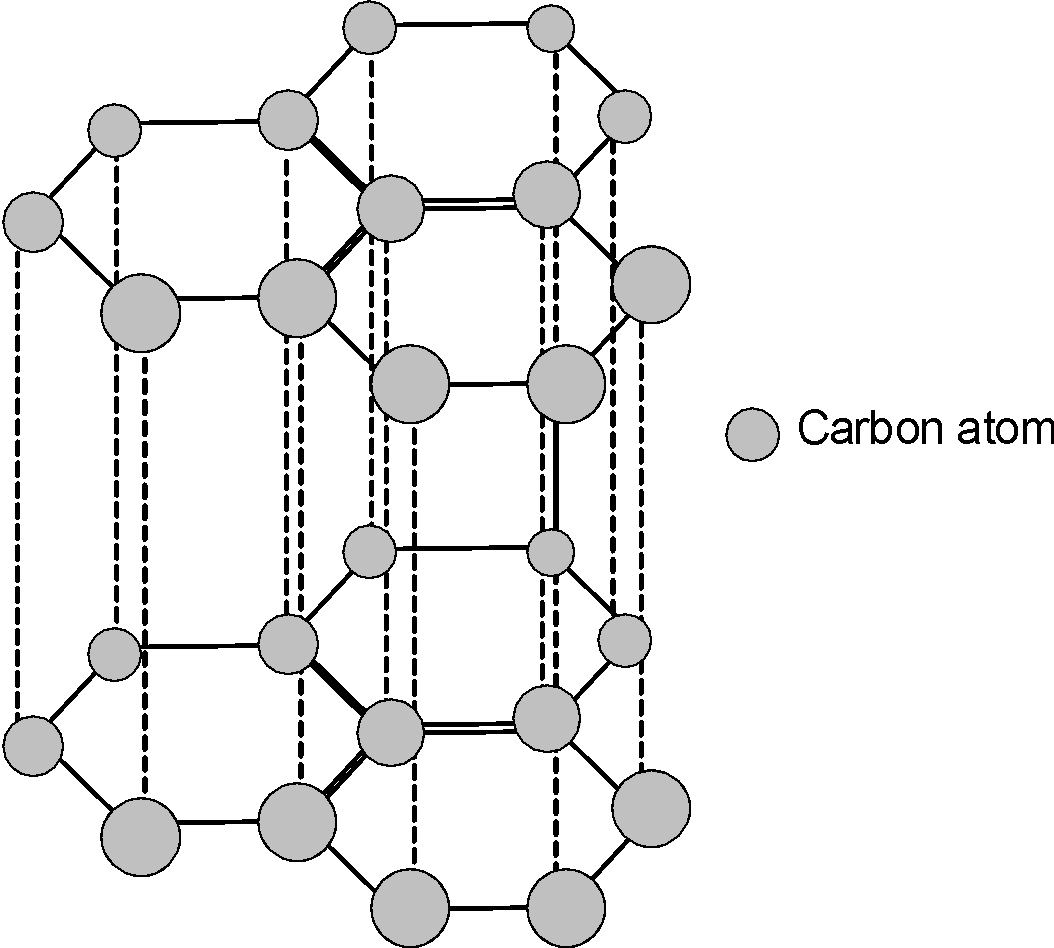
2) Diamond: -It is the pure crystalline allotrope of carbon.
-Each carbon is linked to four neighbouring carbon. The carbon is $\text{s}{{\text{p}}^{\text{3}}}$ hybridized. Thus, carbons are linked tetrahedrally.
-Since the carbons are tetrahedrally arranged, the diamond is a 3-D arrangement of carbon atoms.
-Diamond is an extremely hard allotrope of carbon. This is because the rupturing of a large number of covalent bonds requires a huge amount of energy. This breaking is not an easy task, which makes the diamond the hardest allotropes of carbon on earth.
- Unlike graphite, it is a bad conductor of heat electricity.
Use: The diamonds are hard therefore they are widely used in grinding, cutting, drilling, and polishing of other material.
3) Buckminsterfullerene: -Fullerene is another allotrope of carbon. It was first identified as the ${{\text{C}}_{\text{60}}}$ , in which carbons were arranged in the shape of a football.
-The five and six-membered rings of carbon are arranged to form a ball-like structure. This structure is also known as the ‘Bucky ball’.
Note: It must be noted that ‘allotropes’ of carbon are different from the ‘isomers’ of carbon. Allotropes are the compounds that are strictly made up of the single element but in different chemical formulas as structures. However, the isomers are the chemical compounds made of more than one element that have the same molecular formula but different structural representation.
Complete step by step answer:
First of all, we can check the meaning of the word ‘Allotropy’. The word ‘Allotropy’ is a combination of two words ‘Allos’ meaning ‘other’ and ‘tropos’ meaning ‘form’. So, it has a word meaning ‘other form’.
As the meaning suggests, the term allotrope refers to one or more physical forms of a chemical element that occurs in the same physical state. Allotropes may show differences in chemical and physical properties. The carbon has a property to form a bond with another carbon atom. This is called catenation. Due to this carbon exhibits the allotropy.
Let us have a look at the most widely known allotropes of carbon:
1) Graphite: -It is the purest form of carbon. It is composed of 2-D hexagonal layers of carbon atoms which are arranged in a hexagonal structure.
-Graphite is soft, slippery, and black solid. This property allows the layers of structure to slip over.
-Each carbon in graphite forms three bonds with the adjacent carbon atoms. Therefore, each carbon is $\text{s}{{\text{p}}^{\text{2}}}$ hybridized. The pi-electrons are localized over the structure. Therefore, graphite is a good conductor of electricity.
-Graphite exists in $\text{ }\!\!\alpha\!\!\text{ }$ and $\text{ }\!\!\beta\!\!\text{ }$ forms. In $\text{ }\!\!\alpha\!\!\text{ }$ form, the layers are in sequence of \[\text{ABAB}\] and in $\text{ }\!\!\beta\!\!\text{ }$ form the layers are arranged as\[\text{ABCABC}\] .
Use: It is used to make the lead of a pencil.it is also used as the lubricant.
-The structure of the graphite is as shown below,

2) Diamond: -It is the pure crystalline allotrope of carbon.
-Each carbon is linked to four neighbouring carbon. The carbon is $\text{s}{{\text{p}}^{\text{3}}}$ hybridized. Thus, carbons are linked tetrahedrally.
-Since the carbons are tetrahedrally arranged, the diamond is a 3-D arrangement of carbon atoms.
-Diamond is an extremely hard allotrope of carbon. This is because the rupturing of a large number of covalent bonds requires a huge amount of energy. This breaking is not an easy task, which makes the diamond the hardest allotropes of carbon on earth.
- Unlike graphite, it is a bad conductor of heat electricity.
Use: The diamonds are hard therefore they are widely used in grinding, cutting, drilling, and polishing of other material.

3) Buckminsterfullerene: -Fullerene is another allotrope of carbon. It was first identified as the ${{\text{C}}_{\text{60}}}$ , in which carbons were arranged in the shape of a football.
-The five and six-membered rings of carbon are arranged to form a ball-like structure. This structure is also known as the ‘Bucky ball’.
Note: It must be noted that ‘allotropes’ of carbon are different from the ‘isomers’ of carbon. Allotropes are the compounds that are strictly made up of the single element but in different chemical formulas as structures. However, the isomers are the chemical compounds made of more than one element that have the same molecular formula but different structural representation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




