
What are allotropes? Sketch the structures of two allotropes of carbon namely diamond and graphite. What is the impact of structure on physical properties of two allotropes?
Answer
597k+ views
Hint: Both diamond and graphite have only Carbon atoms in their structure. Graphite is $$s{p^2}$$ hybridized and diamond is $$s{p^3}$$ hybridized. Graphite has a trigonal Planar geometry while diamond has a tetragonal geometry.
Complete Step-by-Step Answer:
Allotropes are each of the forms in which any chemical element can exist.
e.g. Sulfur can exist in both monoclinic and rhombohedral forms which have different physical properties.
Carbon also has two allotropes, namely diamond and graphite.
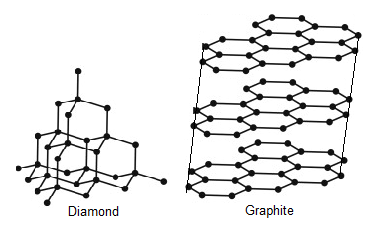
The structures of diamond and graphite are shown in the figure. They have only Carbon atoms in their structure.
Let’s see the effect of their structures on their physical property.
In Diamond, Each Carbon atom is bonded with other 4 carbon atoms. All the Carbon-Carbon bonds are single bonds here. Also, Geometry is Tetragonal having bond angles 109$$^\circ $$28’. So, it gives diamond higher density and very high hardness. Diamonds have a very strong and rigid structure. Diamond is a very good conductor of heat and sound. Diamond cannot conduct electricity. It is chemically inert as well.
Graphite has planar structure and planes can move over one another. It is used in pencils. Graphite is less harder than diamond and also has less density because of its structure. As graphite has a free electron, it lets electricity pass through it. Graphite has lower melting point than diamond because it has planar structure.
Additional Information:
We can see in Diamond that each carbon atom is bonded with 4 carbon atoms and in Graphite, each carbon atom is bonded with other 3 carbon atoms.
Note: Make sure that sketches of diamond and graphite are clear enough and have specific bond angles. While writing about their physical property, also mention what is the reason that they bear that property. There are actually eight known allotropes of carbons available which also includes Fullerene and Nanotubes, so do not get confused with the structures of all of them.
Complete Step-by-Step Answer:
Allotropes are each of the forms in which any chemical element can exist.
e.g. Sulfur can exist in both monoclinic and rhombohedral forms which have different physical properties.
Carbon also has two allotropes, namely diamond and graphite.

The structures of diamond and graphite are shown in the figure. They have only Carbon atoms in their structure.
Let’s see the effect of their structures on their physical property.
In Diamond, Each Carbon atom is bonded with other 4 carbon atoms. All the Carbon-Carbon bonds are single bonds here. Also, Geometry is Tetragonal having bond angles 109$$^\circ $$28’. So, it gives diamond higher density and very high hardness. Diamonds have a very strong and rigid structure. Diamond is a very good conductor of heat and sound. Diamond cannot conduct electricity. It is chemically inert as well.
Graphite has planar structure and planes can move over one another. It is used in pencils. Graphite is less harder than diamond and also has less density because of its structure. As graphite has a free electron, it lets electricity pass through it. Graphite has lower melting point than diamond because it has planar structure.
Additional Information:
We can see in Diamond that each carbon atom is bonded with 4 carbon atoms and in Graphite, each carbon atom is bonded with other 3 carbon atoms.
Note: Make sure that sketches of diamond and graphite are clear enough and have specific bond angles. While writing about their physical property, also mention what is the reason that they bear that property. There are actually eight known allotropes of carbons available which also includes Fullerene and Nanotubes, so do not get confused with the structures of all of them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




