
What are cationic detergents? Give an example.
Answer
583.5k+ views
Hint: Detergents are cleansing agents. They do not form scum with salts in hard water and they are soluble in water. Depending on the electrical charge associated with the detergents there are mainly three types of detergents such as cationic detergent, anionic detergent, and non-ionic detergents. Here we are going to see in detail about cationic detergents.
Complete step by step answer:
Let's begin with the idea of detergents. Detergents are the cleansing agents which can combine with dirt, oil, impurities etc. They increase the wetting and spreading ability of water molecules by decreasing its surface tension.
-For a compound to act as a surface-active agent (detergent, soap etc.) should have some chemical properties. The first one is the presence of a hydrophobic (water fearing) part in the molecule. Generally fatty acids, fatty alcohols, and alkyl benzene act as hydrophobic parts. The second essential condition is the presence of a hydrophilic (water soluble) group such as sulfo groups, -COONa groups etc.
- In short, we can say that the hydrophilic part in detergent attaches to the water and the hydrophobic part attaches to solids or fibres. Based on the electric charge detergent can be classified into cationic, anionic, nonionic and amphoteric detergents.
-Cationic detergents produce electrically positive ions in a solution. They usually contain a long chain cation which is responsible for its surface activities properties. They are usually quaternary ammonium salts of amines with chlorides or bromides, acetates as anions.
- One of the major examples for cationic detergent is cetyltrimethylammonium bromide and its structure is shown below,
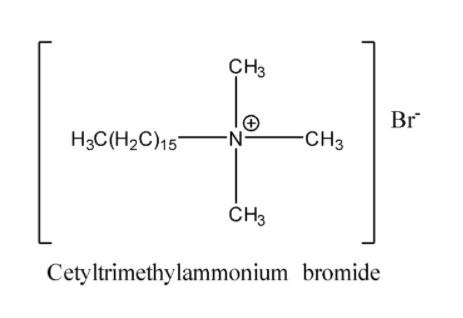
Note: Do not confuse anionic detergents with cationic detergents. The cationic detergents contain a hydrophobic component of quaternary ammonium as the polar end and the ammonium centre is positively charged whereas anionic detergents contain an anion at the soluble end of the long hydrocarbon chain. Also, it should be noted that apart from the cleaning action cationic detergents have good germicidal properties.
Complete step by step answer:
Let's begin with the idea of detergents. Detergents are the cleansing agents which can combine with dirt, oil, impurities etc. They increase the wetting and spreading ability of water molecules by decreasing its surface tension.
-For a compound to act as a surface-active agent (detergent, soap etc.) should have some chemical properties. The first one is the presence of a hydrophobic (water fearing) part in the molecule. Generally fatty acids, fatty alcohols, and alkyl benzene act as hydrophobic parts. The second essential condition is the presence of a hydrophilic (water soluble) group such as sulfo groups, -COONa groups etc.
- In short, we can say that the hydrophilic part in detergent attaches to the water and the hydrophobic part attaches to solids or fibres. Based on the electric charge detergent can be classified into cationic, anionic, nonionic and amphoteric detergents.
-Cationic detergents produce electrically positive ions in a solution. They usually contain a long chain cation which is responsible for its surface activities properties. They are usually quaternary ammonium salts of amines with chlorides or bromides, acetates as anions.
- One of the major examples for cationic detergent is cetyltrimethylammonium bromide and its structure is shown below,

Note: Do not confuse anionic detergents with cationic detergents. The cationic detergents contain a hydrophobic component of quaternary ammonium as the polar end and the ammonium centre is positively charged whereas anionic detergents contain an anion at the soluble end of the long hydrocarbon chain. Also, it should be noted that apart from the cleaning action cationic detergents have good germicidal properties.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




