
What are Chiral centers and chirality?
Answer
517.2k+ views
Hint: Chiral objects are those whose mirror image can’t be superimposed over the original.
In case of hydrocarbons, we say that the mirror image of the $\text{s}{{\text{p}}^{3}}$ hybridized carbon atom and the substituents attached to it have no symmetry.
Complete answer: Chirality- It is the property of a hydrocarbon chain, when its mirror image is not superimposable.
-To check whether the mirror image is superimposable or not, we check for the plane of symmetry.
-Plane of symmetry is an imaginary plane that bisects the molecule, such that the resulting two halves are the identical mirror images of each other.
-If the molecule has a plane of symmetry, it is not chiral. Hence it is called achiral.
-If the molecule does not have a plane of symmetry it is said to be chiral.
-A molecule is always chiral when all the groups attached to the central carbon are different.
-Then the molecule can never have a plane of symmetry as all the groups attached are different and the mirror images could never be identical.
-Hence the molecule is said to be chiral.
-Chiral center: it is the carbon center which has all different groups attached to it in the hydrocarbon chain.
-It is often marked with $''*''$.
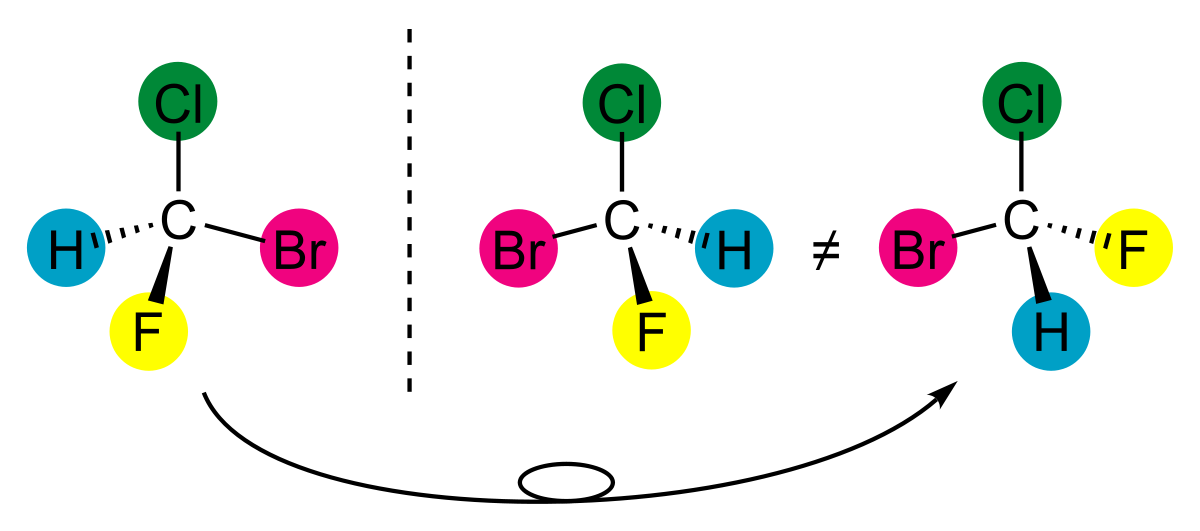
-In the example given above, the carbon atom has four different groups attached to it, namely Cl, H, Br, F.
-The mirror image of this molecule is non superimposable.
Note:
A chiral compound and its mirror images are enantiomers.
Enantiomers are the pair of molecules who are mirror images of each other but cannot be superimposed on each other.
These are a type of stereoisomers (isomers with different spatial arrangements.
In case of hydrocarbons, we say that the mirror image of the $\text{s}{{\text{p}}^{3}}$ hybridized carbon atom and the substituents attached to it have no symmetry.
Complete answer: Chirality- It is the property of a hydrocarbon chain, when its mirror image is not superimposable.
-To check whether the mirror image is superimposable or not, we check for the plane of symmetry.
-Plane of symmetry is an imaginary plane that bisects the molecule, such that the resulting two halves are the identical mirror images of each other.
-If the molecule has a plane of symmetry, it is not chiral. Hence it is called achiral.
-If the molecule does not have a plane of symmetry it is said to be chiral.
-A molecule is always chiral when all the groups attached to the central carbon are different.
-Then the molecule can never have a plane of symmetry as all the groups attached are different and the mirror images could never be identical.
-Hence the molecule is said to be chiral.
-Chiral center: it is the carbon center which has all different groups attached to it in the hydrocarbon chain.
-It is often marked with $''*''$.

-In the example given above, the carbon atom has four different groups attached to it, namely Cl, H, Br, F.
-The mirror image of this molecule is non superimposable.
Note:
A chiral compound and its mirror images are enantiomers.
Enantiomers are the pair of molecules who are mirror images of each other but cannot be superimposed on each other.
These are a type of stereoisomers (isomers with different spatial arrangements.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




