
What are F-centers in an ionic crystal ?
Answer
595.2k+ views
Hint: It occurs in ionic crystals due to crystallographic point defects. More the number of F-centers present in the crystal, higher will be the intensity of color of the crystal.
Complete step-by-step answer:
- F-centers are known as Farbe center. In German the word ‘Farbenzenter’ means a colored center. So, F-centers are responsible for the color property of such ionic crystals which normally are not colored. Now, let’s see the reason behind this phenomenon and details about F-centers.
- Crystal defects may be Stoichiometric or Non-stoichiometric. Non-stoichiometric defects are of two types. (i) Metal excess defect (ii) Metal deficiency defect
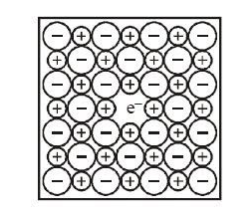
- So, whenever Metal-excess defect is present in the Crystal, it simply means that extra cations are present in the crystal or we can say that anion sites are vacant. Below is the figure of NaCl crystal. We can see a vacant center in the middle in which an anion is missing and an electron has occupied its place.
In a normal NaCl crystal, this type of defect is often not seen, but on heating the crystal in presence of sodium vapour, Chlorine anion in the crystal reacts with the Sodium atom present at the surface and gives an electron. This electron occupies the empty space in the lattice.
- So, when light is allowed to impart on the crystal, the electron gets excited and relaxes to ground state and emits light. In the case of NaCl light emitted is of yellow color.
Additional Information:
- This type of centers are also seen in KCl , LiCl and ZnO crystals.
- Interesting thing about these centers is the fact that they show different colors in different crystals. KCl, LiCl and ZnO show Violet, Pink and Yellow color respectively.
Note:
- All vacant sites do not form F-centers in crystal defects .
- F-centers actually do not contain any atom. It involves only an electron.
Complete step-by-step answer:
- F-centers are known as Farbe center. In German the word ‘Farbenzenter’ means a colored center. So, F-centers are responsible for the color property of such ionic crystals which normally are not colored. Now, let’s see the reason behind this phenomenon and details about F-centers.
- Crystal defects may be Stoichiometric or Non-stoichiometric. Non-stoichiometric defects are of two types. (i) Metal excess defect (ii) Metal deficiency defect
- So, whenever Metal-excess defect is present in the Crystal, it simply means that extra cations are present in the crystal or we can say that anion sites are vacant. Below is the figure of NaCl crystal. We can see a vacant center in the middle in which an anion is missing and an electron has occupied its place.
In a normal NaCl crystal, this type of defect is often not seen, but on heating the crystal in presence of sodium vapour, Chlorine anion in the crystal reacts with the Sodium atom present at the surface and gives an electron. This electron occupies the empty space in the lattice.
- So, when light is allowed to impart on the crystal, the electron gets excited and relaxes to ground state and emits light. In the case of NaCl light emitted is of yellow color.
Additional Information:
- This type of centers are also seen in KCl , LiCl and ZnO crystals.
- Interesting thing about these centers is the fact that they show different colors in different crystals. KCl, LiCl and ZnO show Violet, Pink and Yellow color respectively.
Note:
- All vacant sites do not form F-centers in crystal defects .
- F-centers actually do not contain any atom. It involves only an electron.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




