
What are galvanic cells? Explain the construction and working of galvanic cell with an example?
Answer
582.3k+ views
Hint:A galvanic cell is an electrochemical cell in which there occur some simultaneous chemical changes in order to produce electricity. These chemical changes include several redox reactions which provide a transfer of electrons throughout the cell. Whenever there is a drift in the electrons, current is generated in the opposite direction of the electron drift.
Complete step by step answer:
A galvanic cell or a voltaic cell which is named after the eminent scientists, Luigi Galvani or Alessandro Volta, respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reactions taking place within the cell. It generally comprises two different metals immersed in electrolytes or the circuit of the individual half-cells with different metals and their ions in the solution connected by a salt bridge or separated by a porous membrane. The salt bridge is a U-shaped inverted glass tube like structure consisting of agar solution which and certain electrolytes which join the anodic and cathodic compartments of the electrolytic cell and eases the smooth movement of electrons through it.
An example of a galvanic cell is the Daniel cell. Let us understand the construction and working of the Daniel cell.
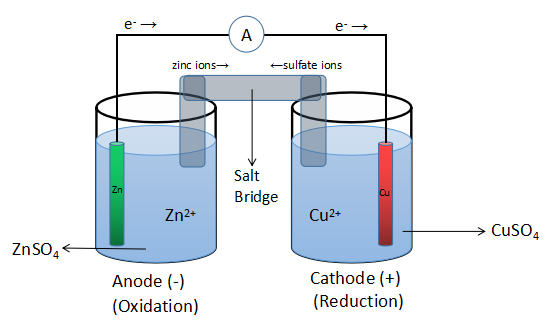
Construction: The Daniel cell has a zinc electrode dipped in a solution of zinc sulfate and forms the anode (negative electrode). It has a copper electrode dipped in a solution of copper sulfate and forms the anode (positive electrode) of the cell. Both the electrodes are connected to each other by two ways: internally and externally. The anode is connected to the cathode externally with the help of a wire and internally with the help of the salt bridge.
Working: There is a flow of current in the external circuit from anode to cathode and in order to compensate this loss of negative charge, the zinc ions in the anode start moving towards the cathode internally with the help of the salt bridge. At this potential barrier, the sulfate ions start moving from cathode to the anode to maintain the electronic neutrality across this barrier. Thus, at anode, oxidation of zinc electrode occurs and at cathode, reduction of copper ions takes place. The reaction that takes place here is:
$Z{n^{2 + }}(aq) + Cu(s) \to Zn(s) + C{u^{2 + }}(aq)$
Note:The transfer of electrons in the external circuit from anode to cathode generates an electric current from cathode to anode (opposite to the flow of electrons). Galvanic cells play a crucial role in our day to day lives for providing electric current of small power scale.
Complete step by step answer:
A galvanic cell or a voltaic cell which is named after the eminent scientists, Luigi Galvani or Alessandro Volta, respectively, is an electrochemical cell that derives electrical energy from spontaneous redox reactions taking place within the cell. It generally comprises two different metals immersed in electrolytes or the circuit of the individual half-cells with different metals and their ions in the solution connected by a salt bridge or separated by a porous membrane. The salt bridge is a U-shaped inverted glass tube like structure consisting of agar solution which and certain electrolytes which join the anodic and cathodic compartments of the electrolytic cell and eases the smooth movement of electrons through it.
An example of a galvanic cell is the Daniel cell. Let us understand the construction and working of the Daniel cell.

Construction: The Daniel cell has a zinc electrode dipped in a solution of zinc sulfate and forms the anode (negative electrode). It has a copper electrode dipped in a solution of copper sulfate and forms the anode (positive electrode) of the cell. Both the electrodes are connected to each other by two ways: internally and externally. The anode is connected to the cathode externally with the help of a wire and internally with the help of the salt bridge.
Working: There is a flow of current in the external circuit from anode to cathode and in order to compensate this loss of negative charge, the zinc ions in the anode start moving towards the cathode internally with the help of the salt bridge. At this potential barrier, the sulfate ions start moving from cathode to the anode to maintain the electronic neutrality across this barrier. Thus, at anode, oxidation of zinc electrode occurs and at cathode, reduction of copper ions takes place. The reaction that takes place here is:
$Z{n^{2 + }}(aq) + Cu(s) \to Zn(s) + C{u^{2 + }}(aq)$
Note:The transfer of electrons in the external circuit from anode to cathode generates an electric current from cathode to anode (opposite to the flow of electrons). Galvanic cells play a crucial role in our day to day lives for providing electric current of small power scale.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




