
What are some examples of molecular orbitals?
Answer
486k+ views
Hint: In chemistry, chemical bonds are three types of classification and they are covalent bonds, ionic bonds, and coordinate bonds. This is based on electron binding in the bond. The covalent bond is the mutual sharing of electrons between the two atoms in the molecule. The ionic bond is nothing but highly electronegativity pulls the electrons towards itself, at least the electronegativity atom loses the electrons in the molecule. The coordinate bond is nothing but a pair of electrons from one atom to another atom in the molecule.
Complete answer:
The mutual sharing of electrons between the two atoms in the molecule is called the covalent bond.
The atomic orbitals are overlapping to give molecular orbitals. The number of atomic orbitals and the number of molecular orbitals formed will be equal.
An example of molecular orbitals is hydrogen molecules.
The number of bonds in a hydrogen molecule is one and the number of an electron is two.
The molecular orbital diagram of the hydrogen molecule is given below,
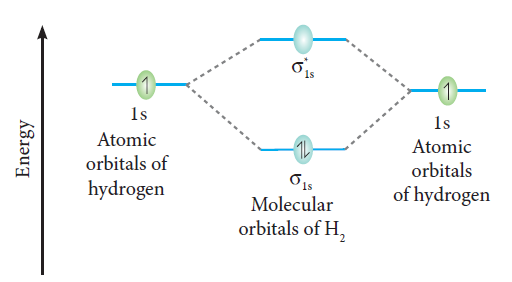
The bond order of the hydrogen molecule is one.
Note:
The covalent bond is important for organic chemistry. In that mutual sharing of electrons in the atom, some overlapping of orbitals are also involved in the covalent bond. This overlapping of orbitals involves the mutual sharing of electrons in the atom. These overlapping are classified as two types are head-wise overlapping and sidewise overlapping. There are two types of covalent bonds. There are sigma bonds and pi bonds. The sigma bond forms because of the head-wise overlapping of the atomic orbital in the molecular orbitals. The pi bond forms because of the side-wise overlapping of the atomic orbitals in the molecular orbitals.
Complete answer:
The mutual sharing of electrons between the two atoms in the molecule is called the covalent bond.
The atomic orbitals are overlapping to give molecular orbitals. The number of atomic orbitals and the number of molecular orbitals formed will be equal.
An example of molecular orbitals is hydrogen molecules.
The number of bonds in a hydrogen molecule is one and the number of an electron is two.
The molecular orbital diagram of the hydrogen molecule is given below,

The bond order of the hydrogen molecule is one.
Note:
The covalent bond is important for organic chemistry. In that mutual sharing of electrons in the atom, some overlapping of orbitals are also involved in the covalent bond. This overlapping of orbitals involves the mutual sharing of electrons in the atom. These overlapping are classified as two types are head-wise overlapping and sidewise overlapping. There are two types of covalent bonds. There are sigma bonds and pi bonds. The sigma bond forms because of the head-wise overlapping of the atomic orbital in the molecular orbitals. The pi bond forms because of the side-wise overlapping of the atomic orbitals in the molecular orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




