
What are the 3 types of thermodynamic systems?
Answer
503.7k+ views
Hint: According to thermodynamics a system is defined as an element of the universe which is under the observation process. Thermodynamic system may be homogeneous or heterogeneous depending upon the total number of phases constituting the system.
Complete answer:
Thermodynamic systems are classified on the basis of exchange or movement of energy and matter into the system or out of the system.
Types of thermodynamic systems-
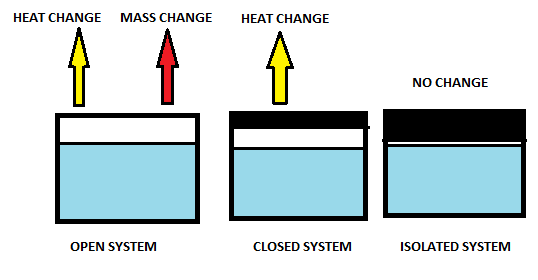
Open system
Closed system
Isolated system
Open system is one of the thermodynamic systems which allow the exchange of matter and energy both simultaneously with the surrounding.
For example, chemical reaction between the sodium sulphite and dilute hydrochloric acid in the test tube results in the formation of sodium chloride salt, water and sulphur dioxide gas. This reaction results in evolution of sulphur dioxide gas along with generation of some heat during the process.
$N{a_2}S{O_3} + 2HCl \to 2NaCl + {H_2}O + S{O_2} \uparrow $
Hence, from the above example we see that there is change of both matter and energy taking place and it forms the open system.
Closed system allows only the exchange of energy but does not allow exchange of matter from the system.
For example, the chemical reaction of hydrogen iodide in the presence of heat under a closed chamber will lead to formation of hydrogen gas and iodine.
$2HI \to {H_2} \uparrow + 2I$
Hence, from the above example we see that there is change of energy only while matter of reaction is not exchanged and it forms the closed system.
Chemical reactions which are carried out in a closed chamber are good examples of closed systems.
Isolated system does not allow transfer of matter and energy of the system with surroundings.
Practically it is difficult to design such a system which is an isolated but sealed chamber which does not have any option of matter input or output and act as a thermally isolated body to maintain the temperature which prevents heat flow are considered an isolated system.
For example, reactions of radioactive compounds which are performed in a chamber which is completely sealed and isolated from the surrounding is an example of an isolated system.
Note:
Systems and surroundings collectively form the universe. Surrounding is defined as part of the universe which is not a part of the system.
All the chemical reactions and physical reactions that take place in our day to day life are examples of an open system.
Complete answer:
Thermodynamic systems are classified on the basis of exchange or movement of energy and matter into the system or out of the system.
Types of thermodynamic systems-
Open system
Closed system
Isolated system
Open system is one of the thermodynamic systems which allow the exchange of matter and energy both simultaneously with the surrounding.
For example, chemical reaction between the sodium sulphite and dilute hydrochloric acid in the test tube results in the formation of sodium chloride salt, water and sulphur dioxide gas. This reaction results in evolution of sulphur dioxide gas along with generation of some heat during the process.
$N{a_2}S{O_3} + 2HCl \to 2NaCl + {H_2}O + S{O_2} \uparrow $
Hence, from the above example we see that there is change of both matter and energy taking place and it forms the open system.
Closed system allows only the exchange of energy but does not allow exchange of matter from the system.
For example, the chemical reaction of hydrogen iodide in the presence of heat under a closed chamber will lead to formation of hydrogen gas and iodine.
$2HI \to {H_2} \uparrow + 2I$
Hence, from the above example we see that there is change of energy only while matter of reaction is not exchanged and it forms the closed system.
Chemical reactions which are carried out in a closed chamber are good examples of closed systems.
Isolated system does not allow transfer of matter and energy of the system with surroundings.
Practically it is difficult to design such a system which is an isolated but sealed chamber which does not have any option of matter input or output and act as a thermally isolated body to maintain the temperature which prevents heat flow are considered an isolated system.
For example, reactions of radioactive compounds which are performed in a chamber which is completely sealed and isolated from the surrounding is an example of an isolated system.

Note:
Systems and surroundings collectively form the universe. Surrounding is defined as part of the universe which is not a part of the system.
All the chemical reactions and physical reactions that take place in our day to day life are examples of an open system.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




