
What are the 9 isomers of Heptane?
Answer
510k+ views
Hint: Isomers are those compounds which have the same molecular formula but have different spatial arrangement. Heptane is an alkane having seven carbon atoms. Hence, the chemical formula for heptane is \[C_7H_{16}\].
Complete answer:
The chemical formula for heptane is \[C7H16\]. The structure of first isomer named as n-heptane is given as follows:
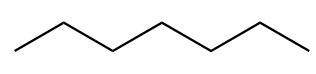
The structure of second isomer named as 2-Methylhexane is given as follows:
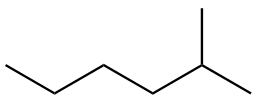
The structure of third isomer named as 3-Methylhexane is given as follows:
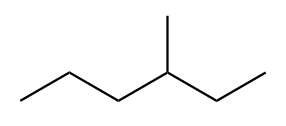
The structure of fourth isomer named as 2,2-Dimethylpentane is given as follows:
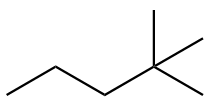
The structure of fifth isomer named as 2,3-Dimethylpentane is given as follows:
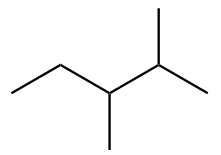
The structure of sixth isomer named as 2,4-Dimethylpentane is given as follows:
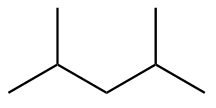
The structure of seventh isomer named as 3,3-Dimethylpentane is given as follows:
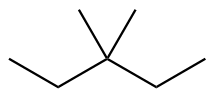
The structure of eighth isomer named as 3-Ethylpentane is given as follows:
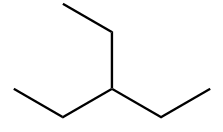
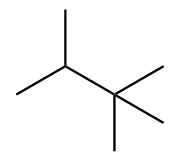
The structure of ninth isomer named as 2,2,3-Trimethylbutane is given as follows:
From the above structures, we can see that all structures differ in structural arrangement in space and have the same molecular formula.
Therefore, the 9 isomers of heptane are n-heptane, 2-Methylhexane, 3-Methylhexane, 2,2-Dimethylpentane, 2,3-Dimethylpentane, 2,4-Dimethylpentane, 3,3-Dimethylpentane, 3-Ethylpentane and 2,2,3-Trimethylbutane.
Note:
It is important to note that isomers have the same chemical formula but have different structures. The 9 isomers of heptane differ in the number of carbon atoms in the parent chain. The IUPAC name of these isomers are n-heptane, 2-Methylhexane, 3-Methylhexane, 2,2-Dimethylpentane, 2,3-Dimethylpentane, 2,4-Dimethylpentane, 3,3-Dimethylpentane, 3-Ethylpentane and 2,2,3-Trimethylbutane.
Complete answer:
The chemical formula for heptane is \[C7H16\]. The structure of first isomer named as n-heptane is given as follows:

The structure of second isomer named as 2-Methylhexane is given as follows:

The structure of third isomer named as 3-Methylhexane is given as follows:

The structure of fourth isomer named as 2,2-Dimethylpentane is given as follows:

The structure of fifth isomer named as 2,3-Dimethylpentane is given as follows:

The structure of sixth isomer named as 2,4-Dimethylpentane is given as follows:

The structure of seventh isomer named as 3,3-Dimethylpentane is given as follows:

The structure of eighth isomer named as 3-Ethylpentane is given as follows:

The structure of ninth isomer named as 2,2,3-Trimethylbutane is given as follows:

From the above structures, we can see that all structures differ in structural arrangement in space and have the same molecular formula.
Therefore, the 9 isomers of heptane are n-heptane, 2-Methylhexane, 3-Methylhexane, 2,2-Dimethylpentane, 2,3-Dimethylpentane, 2,4-Dimethylpentane, 3,3-Dimethylpentane, 3-Ethylpentane and 2,2,3-Trimethylbutane.
Note:
It is important to note that isomers have the same chemical formula but have different structures. The 9 isomers of heptane differ in the number of carbon atoms in the parent chain. The IUPAC name of these isomers are n-heptane, 2-Methylhexane, 3-Methylhexane, 2,2-Dimethylpentane, 2,3-Dimethylpentane, 2,4-Dimethylpentane, 3,3-Dimethylpentane, 3-Ethylpentane and 2,2,3-Trimethylbutane.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




