
What are the chemical effects of electric current? Explain the process of electroplating.
Answer
487.8k+ views
Hint: Whenever an electric current is passed through a conducting solution then due to this some reaction takes place inside the solution which is known as the chemical effects of electric current. Due to these chemical reactions we can add a layer of metal over other metal which is the concept of electroplating. We will discuss electroplating as one of the applications of the chemical effect of electric current.
Complete answer:
When electric current is passed through an aqueous solution of a substance then the constituent ions get broken into cations and anions. Since the number of ions increases, the conductivity of the solution also increases.
The chemical effects which can be seen with the passage of electric current in the aqueous solution are listed below as:
A. Formation of gas bubbles may take place at electrodes due to chemical reactions taking place in the solution.
B. Metals being deposited at the electrode after the oxidation and reduction process.
C. There might be a change in color of the solution.
Electroplating: Electroplating may be defined as the process when a certain metal is being coated over another metal. This coating or plating of metal takes place during the electrolysis of the solution. Thus it is known as electroplating. For example: If we want an electroplating of copper over the brass electrode then we have to need a copper electrode, a brass electrode and a solution or electrolyte which contains copper ions in it. The whole process can be seen in the below figure.
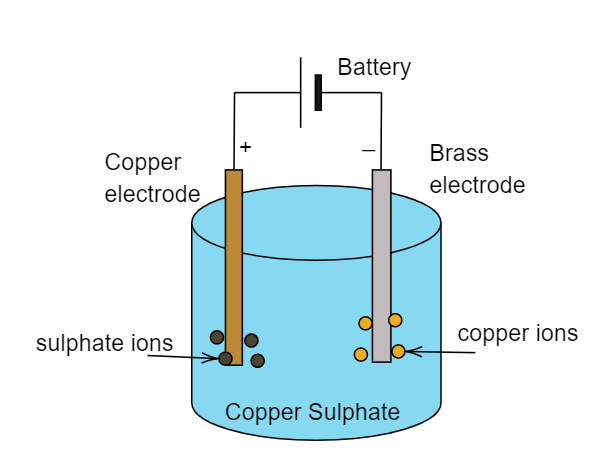
Note:
Electrolyte is the solution which dissociates into its consecutive ions when electric current is passed through it. Here electrolytes are copper sulphates. Electroplating is also used for preventing metal from corrosion and it is an extensively used process in automobile industries. The conduction of solutions increases as the number of ions increases.
Complete answer:
When electric current is passed through an aqueous solution of a substance then the constituent ions get broken into cations and anions. Since the number of ions increases, the conductivity of the solution also increases.
The chemical effects which can be seen with the passage of electric current in the aqueous solution are listed below as:
A. Formation of gas bubbles may take place at electrodes due to chemical reactions taking place in the solution.
B. Metals being deposited at the electrode after the oxidation and reduction process.
C. There might be a change in color of the solution.
Electroplating: Electroplating may be defined as the process when a certain metal is being coated over another metal. This coating or plating of metal takes place during the electrolysis of the solution. Thus it is known as electroplating. For example: If we want an electroplating of copper over the brass electrode then we have to need a copper electrode, a brass electrode and a solution or electrolyte which contains copper ions in it. The whole process can be seen in the below figure.

Note:
Electrolyte is the solution which dissociates into its consecutive ions when electric current is passed through it. Here electrolytes are copper sulphates. Electroplating is also used for preventing metal from corrosion and it is an extensively used process in automobile industries. The conduction of solutions increases as the number of ions increases.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




