
What are the chiral centers in sucralose?
Answer
492.6k+ views
Hint: Sucralose is a disaccharide derivative consisting of two simple carbohydrate units linked by a glycosidic bond. The molecular formula of sucralose is $ {C_{12}}{H_{19}}C{l_3}{O_8} $ . The chiral centre means the carbon with four different groups. They are a total of $ 9 $ chiral centres in sucralose.
Complete answer:
Sucralose is a disaccharide derivative, disaccharide means upon hydrolysis, it will give two simple molecules of monosaccharide units like glucose. Sucrose is also an example of disaccharide. The molecular formula of sucralose is $ {C_{12}}{H_{19}}C{l_3}{O_8} $ . whereas the molecular formula of sucrose is $ {C_{11}}{H_{22}}{O_{11}} $ .
Chiral centre means the carbon attached to four different groups. By looking at the structure of sucralose, the chiral centres can be known.
The structure of sucralose is
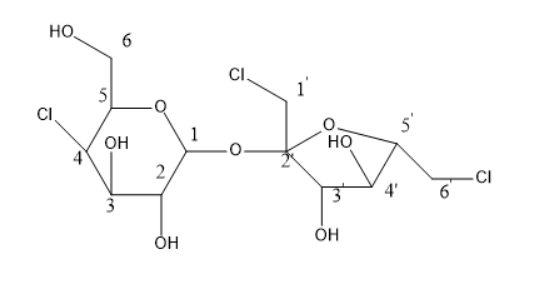
In the above structure, there is a total of $ 12 $ carbons. Out of $ 12 $ carbons, $ {1'} $ has two hydrogen atoms, $ {6'} $ has two hydrogen atoms and $ 6 $ carbon has also two hydrogen atoms. Thus, these three carbon centres are not chiral. Whereas the remaining carbons are all chiral centres.
Thus, the chiral centres are $ 1,2,3,4,5,{2'},{3'},{4'}, $ and $ {5'} $ . As these all carbons have four different groups attached with them and are known as chiral centres.
The chiral centres in sucralose are $ 9 $ and these are $ 1,2,3,4,5,{2'},{3'},{4'}, $ and $ {5'} $ position carbon atoms.
Note:
Chiral centres were important to determine the optical activity and also in the determination of configuration of alkanes containing four different groups which is known as chirality. The Entgegen (E) and Zusammen (Z) configuration can be written to the alkanes with chirality.
Complete answer:
Sucralose is a disaccharide derivative, disaccharide means upon hydrolysis, it will give two simple molecules of monosaccharide units like glucose. Sucrose is also an example of disaccharide. The molecular formula of sucralose is $ {C_{12}}{H_{19}}C{l_3}{O_8} $ . whereas the molecular formula of sucrose is $ {C_{11}}{H_{22}}{O_{11}} $ .
Chiral centre means the carbon attached to four different groups. By looking at the structure of sucralose, the chiral centres can be known.
The structure of sucralose is

In the above structure, there is a total of $ 12 $ carbons. Out of $ 12 $ carbons, $ {1'} $ has two hydrogen atoms, $ {6'} $ has two hydrogen atoms and $ 6 $ carbon has also two hydrogen atoms. Thus, these three carbon centres are not chiral. Whereas the remaining carbons are all chiral centres.
Thus, the chiral centres are $ 1,2,3,4,5,{2'},{3'},{4'}, $ and $ {5'} $ . As these all carbons have four different groups attached with them and are known as chiral centres.
The chiral centres in sucralose are $ 9 $ and these are $ 1,2,3,4,5,{2'},{3'},{4'}, $ and $ {5'} $ position carbon atoms.
Note:
Chiral centres were important to determine the optical activity and also in the determination of configuration of alkanes containing four different groups which is known as chirality. The Entgegen (E) and Zusammen (Z) configuration can be written to the alkanes with chirality.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




