
What are the condensed structural formulae for the following molecules
A. Methanoic acid
B. cis-But-2-ene
C. Cyclopentanol
D. 3-methylcyclohexene
Answer
494.1k+ views
Hint: A condensed structural formula is a system of representing organic structures in a line of text which shows all atoms but omits the vertical bonds and almost all the horizontal single bonds. When a group of atoms are bonded to a single atom in the compound, then parenthesis is used around the group along with the carbon atom to which it is bonded.
Complete answer:
The condensed structural formulae for each given molecule are as follows:
A. Methanoic acid: It is also known as formic acid and it is the simplest carboxylic acid with molecular formula $ C{O_2}{H_2} $ . The condensed structural formula of methanoic acid is $ HCOOH $ .
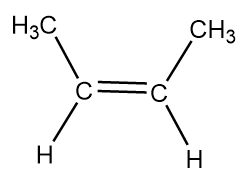
B. cis-But-2-ene: The molecular formula of butene is $ {C_4}{H_8} $ and in order to show its cis form, we need to all horizontal bonds within the structural formula. So, instead of representing it with condensed structural formula, it is preferred to represent the stereochemistry of the molecule with general structural formula which is as follows:
C. Cyclopentanol: The molecular formula of Cyclopentanol is $ {C_5}{H_{10}}O $ and because of the presence of a ring, we cannot represent the molecule in a condensed structural formula. The general structural formula of Cyclopentanol is as follows:

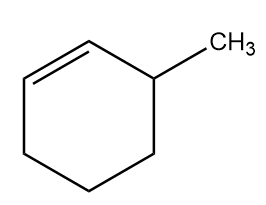
D. 3-methylcyclohexene: The molecular formula of 3-methylcyclohexene is $ {C_7}{H_{12}} $ and because of the presence of ring, we cannot represent the molecule in condensed structural formula. The general structural formula of 3-methylcyclohexene is as follows:
Note:
Remember that the structural formula is preferred over the molecular formula for representing organic compounds because in most of the cases a molecular formula does not uniquely represent a single compound but may represent a group of molecules having different structural formulae known as isomers.
Complete answer:
The condensed structural formulae for each given molecule are as follows:
A. Methanoic acid: It is also known as formic acid and it is the simplest carboxylic acid with molecular formula $ C{O_2}{H_2} $ . The condensed structural formula of methanoic acid is $ HCOOH $ .
B. cis-But-2-ene: The molecular formula of butene is $ {C_4}{H_8} $ and in order to show its cis form, we need to all horizontal bonds within the structural formula. So, instead of representing it with condensed structural formula, it is preferred to represent the stereochemistry of the molecule with general structural formula which is as follows:

C. Cyclopentanol: The molecular formula of Cyclopentanol is $ {C_5}{H_{10}}O $ and because of the presence of a ring, we cannot represent the molecule in a condensed structural formula. The general structural formula of Cyclopentanol is as follows:

D. 3-methylcyclohexene: The molecular formula of 3-methylcyclohexene is $ {C_7}{H_{12}} $ and because of the presence of ring, we cannot represent the molecule in condensed structural formula. The general structural formula of 3-methylcyclohexene is as follows:

Note:
Remember that the structural formula is preferred over the molecular formula for representing organic compounds because in most of the cases a molecular formula does not uniquely represent a single compound but may represent a group of molecules having different structural formulae known as isomers.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




